Changes in Synaptic Proteins Precede Neurodegeneration Markers in Preclinical Alzheimer's Disease Cerebrospinal Fluid
- PMID: 30606734
- PMCID: PMC6398205
- DOI: 10.1074/mcp.RA118.001290
Changes in Synaptic Proteins Precede Neurodegeneration Markers in Preclinical Alzheimer's Disease Cerebrospinal Fluid
Abstract
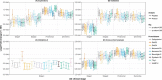
A biomarker of synapse loss, an early event in Alzheimer's disease (AD) pathophysiology that precedes neuronal death and symptom onset, would be a much-needed prognostic biomarker. With direct access to the brain interstitial fluid, the cerebrospinal fluid (CSF) is a potential source of synapse-derived proteins. In this study, we aimed to identify and validate novel CSF biomarkers of synapse loss in AD. Discovery: Combining shotgun proteomics of the CSF with an exhaustive search of the literature and public databases, we identified 251 synaptic proteins, from which we selected 22 for further study. Verification: Twelve proteins were discarded because of poor detection by Selected Reaction Monitoring (SRM). We confirmed the specific expression of 9 of the remaining proteins (Calsynytenin-1, GluR2, GluR4, Neurexin-2A, Neurexin-3A, Neuroligin-2, Syntaxin-1B, Thy-1, Vamp-2) at the human synapse using Array Tomography microscopy and biochemical fractionation methods. Exploration: Using SRM, we monitored these 9 synaptic proteins (20 peptides) in a cohort of CSF from cognitively normal controls and subjects in the pre-clinical and clinical AD stages (n = 80). Compared with controls, peptides from 8 proteins were elevated 1.3 to 1.6-fold (p < 0.04) in prodromal AD patients. Validation: Elevated levels of a GluR4 peptide at the prodromal stage were replicated (1.3-fold, p = 0.04) in an independent cohort (n = 60). Moreover, 7 proteins were reduced at preclinical stage 1 (0.6 to 0.8-fold, p < 0.04), a finding that was replicated (0.7 to 0.8-fold, p < 0.05) for 6 proteins in a third cohort (n = 38). In a cross-cohort meta-analysis, 6 synaptic proteins (Calsyntenin-1, GluR4, Neurexin-2A, Neurexin-3A, Syntaxin-1B and Thy-1) were reduced 0.8-fold (p < 0.05) in preclinical AD, changes that precede clinical symptoms and CSF markers of neurodegeneration. Therefore, these proteins could have clinical value for assessing disease progression, especially in preclinical stages of AD.
Keywords: Alzheimer's disease; Biomarker: Prognostic; Cerebrospinal fluid; Clinical proteomics; Selected reaction monitoring.
© 2019 Lleó et al.
Conflict of interest statement
The authors declare that the Biomedical Research Institute Sant Pau (IIB Sant Pau) has filed a patent application (pending) to the European Patent Office (EP18382175.0) to protect the intellectual property included in this manuscript. O Belbin, A Lleó, Á Bayés, J Fortea and D Alcolea are the named inventors
Figures


References
-
- Selkoe D. J. (2002) Alzheimer's disease is a synaptic failure. Science 298, 789–791 - PubMed
-
- Calabrese F., Riva M. A., and Molteni R. (2016) Synaptic alterations associated with depression and schizophrenia: potential as a therapeutic target. Expert. Opin. Ther. Targets 20, 1195–1207 - PubMed
-
- Bellucci A., Mercuri N. B., Venneri A., Faustini G., Longhena F., Pizzi M., Missale C., and Spano P. (2016) Review: Parkinson's disease: from synaptic loss to connectome dysfunction. Neuropathol. Appl. Neurobiol. 42, 77–94 - PubMed
-
- Albert M. S., DeKosky S. T., Dickson D., Dubois B., Feldman H. H., Fox N. C., Gamst A., Holtzman D. M., Jagust W. J., Petersen R. C., Snyder P. J., Carrillo M. C., Thies B., and Phelps C. H. (2011) The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7, 270–279 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

