A Distinct Visual Pathway Mediates High-Intensity Light Adaptation of the Circadian Clock in Drosophila
- PMID: 30606757
- PMCID: PMC6391560
- DOI: 10.1523/JNEUROSCI.1497-18.2018
A Distinct Visual Pathway Mediates High-Intensity Light Adaptation of the Circadian Clock in Drosophila
Abstract
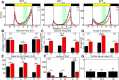
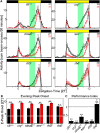
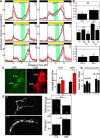
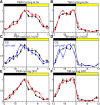
To provide organisms with a fitness advantage, circadian clocks have to react appropriately to changes in their environment. High-intensity (HI) light plays an essential role in the adaptation to hot summer days, which especially endanger insects of desiccation or prey visibility. Here, we show that solely increasing light intensity leads to an increased midday siesta in Drosophila behavior. Interestingly, this change is independent of the fly's circadian photoreceptor cryptochrome and is solely caused by a small visual organ, the Hofbauer-Buchner eyelets. Using receptor knock-downs, immunostaining, and recently developed calcium tools, we show that the eyelets activate key core clock neurons, namely the s-LNvs, at HI. This activation delays the decrease of PERIOD (PER) in the middle of the day and propagates to downstream target clock neurons that prolong the siesta. We show a new pathway for integrating light-intensity information into the clock network, suggesting new network properties and surprising parallels between Drosophila and the mammalian system.SIGNIFICANCE STATEMENT The ability of animals to adapt to their ever-changing environment plays an important role in their fitness. A key player in this adaptation is the circadian clock. For animals to predict the changes of day and night, they must constantly monitor, detect and incorporate changes in the environment. The appropriate incorporation and reaction to high-intensity (HI) light is of special importance for insects because they might suffer from desiccation during hot summer days. We show here that different photoreceptors have specialized functions to integrate low-intensity, medium-intensity, or HI light into the circadian system in Drosophila These results show surprising parallels to mammalian mechanisms, which also use different photoreceptor subtypes to respond to different light intensities.
Keywords: Drosophila; circadian clock; photoreceptor.
Copyright © 2019 the authors 0270-6474/19/391621-10$15.00/0.
Figures

Similar articles
-
A Neural Network Underlying Circadian Entrainment and Photoperiodic Adjustment of Sleep and Activity in Drosophila.J Neurosci. 2016 Aug 31;36(35):9084-96. doi: 10.1523/JNEUROSCI.0992-16.2016. J Neurosci. 2016. PMID: 27581451 Free PMC article.
-
A Tug-of-War between Cryptochrome and the Visual System Allows the Adaptation of Evening Activity to Long Photoperiods in Drosophila melanogaster.J Biol Rhythms. 2018 Feb;33(1):24-34. doi: 10.1177/0748730417738612. Epub 2017 Nov 28. J Biol Rhythms. 2018. PMID: 29179610
-
Light input pathways to the circadian clock of insects with an emphasis on the fruit fly Drosophila melanogaster.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020 Mar;206(2):259-272. doi: 10.1007/s00359-019-01379-5. Epub 2019 Nov 5. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020. PMID: 31691095 Free PMC article. Review.
-
Cryptochrome-dependent and -independent circadian entrainment circuits in Drosophila.J Neurosci. 2015 Apr 15;35(15):6131-41. doi: 10.1523/JNEUROSCI.0070-15.2015. J Neurosci. 2015. PMID: 25878285 Free PMC article.
-
A fly's eye view of circadian entrainment.J Biol Rhythms. 2003 Jun;18(3):206-16. doi: 10.1177/0748730403018003003. J Biol Rhythms. 2003. PMID: 12828278 Review.
Cited by
-
Entrainment of the Drosophila clock by the visual system.Neurosci Insights. 2020 Feb 5;15:2633105520903708. doi: 10.1177/2633105520903708. eCollection 2020. Neurosci Insights. 2020. PMID: 35174330 Free PMC article. Review.
-
Role of Rhodopsins as Circadian Photoreceptors in the Drosophila melanogaster.Biology (Basel). 2019 Jan 10;8(1):6. doi: 10.3390/biology8010006. Biology (Basel). 2019. PMID: 30634679 Free PMC article. Review.
-
Age-dependent effects of blue light exposure on lifespan, neurodegeneration, and mitochondria physiology in Drosophila melanogaster.NPJ Aging. 2022 Jul 27;8(1):11. doi: 10.1038/s41514-022-00092-z. NPJ Aging. 2022. PMID: 35927421 Free PMC article.
-
Mutual coupling of neurons in the circadian master clock: What we can learn from fruit flies.Neurobiol Sleep Circadian Rhythms. 2025 Jan 17;18:100112. doi: 10.1016/j.nbscr.2025.100112. eCollection 2025 May. Neurobiol Sleep Circadian Rhythms. 2025. PMID: 39906412 Free PMC article.
-
Reorganization of circadian activity and the pacemaker circuit under novel light regimes.Proc Biol Sci. 2024 Aug;291(2027):20241190. doi: 10.1098/rspb.2024.1190. Epub 2024 Jul 24. Proc Biol Sci. 2024. PMID: 39043245 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
