Acquired Expression of Mutant Mitofusin 2 Causes Progressive Neurodegeneration and Abnormal Behavior
- PMID: 30606759
- PMCID: PMC6391572
- DOI: 10.1523/JNEUROSCI.2139-18.2018
Acquired Expression of Mutant Mitofusin 2 Causes Progressive Neurodegeneration and Abnormal Behavior
Abstract
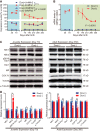
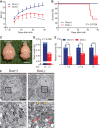
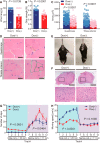
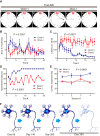
Neurons have high plasticity in developmental and juvenile stages that decreases in adulthood. Mitochondrial dynamics are highly important in neurons to maintain normal function. To compare dependency on mitochondrial dynamics in juvenile and adult stages, we generated a mouse model capable of selective timing of the expression of a mutant of the mitochondrial fusion factor Mitofusin 2 (MFN2). Mutant expression in the juvenile stage had lethal effects. Contrastingly, abnormalities did not manifest until 150 d after mutant expression during adulthood. After this silent 150 d period, progressive neurodegeneration, abnormal behaviors, and learning and memory deficits similar to those seen in human neurodegenerative diseases were observed. This indicates that abnormal neuronal mitochondrial dynamics seriously affect survival during early life stages and can also significantly damage brain function after maturation. Our findings highlight the need to consider the timing of disease onset in mimicking human neurodegenerative diseases.SIGNIFICANCE STATEMENT To compare the dependency on mitochondrial dynamics in neurons in juvenile and adult stages, we generated a mouse model expressing a mutant of the mitochondrial fusion factor MFN2 in an arbitrary timing. Juvenile expression of the mutant showed acute and severe phenotypes and had lethal effects; however, post-adult expression induced delayed but progressive phenotypes resembling those found in human neurodegenerative diseases. Our results indicate that abnormal neuronal mitochondrial dynamics seriously affect survival during early life stages and can also significantly damage brain function after maturation. This strongly suggests that the timing of expression should be considered when establishing an animal model that closely resembles human neurodegenerative diseases.
Keywords: MFN2; cognitive impairment; hyperactivity; mitochondrial dynamics; progressive neurodegeneration.
Copyright © 2019 the authors 0270-6474/19/391588-17$15.00/0.
Figures





References
-
- Ahlemeyer B, Halupczok S, Rodenberg-Frank E, Valerius KP, Baumgart-Vogt E (2018) Endogenous murine amyloid-beta peptide assembles into aggregates in the aged C57BL/6J mouse suggesting these animals as a model to study pathogenesis of amyloid-beta plaque formation. J Alzheimers Dis 61:1425–1450. 10.3233/JAD-170923 - DOI - PubMed
-
- Baek SH, Park SJ, Jeong JI, Kim SH, Han J, Kyung JW, Baik SH, Choi Y, Choi BY, Park JS, Bahn G, Shin JH, Jo DS, Lee JY, Jang CG, Arumugam TV, Kim J, Han JW, Koh JY, Cho DH, Jo DG (2017) Inhibition of Drp1 ameliorates synaptic depression, Aβ deposition, and cognitive impairment in an Alzheimer's disease model. J Neurosci 37:5099–5110. 10.1523/JNEUROSCI.2385-16.2017 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
