A Randomized Trial of Zoledronic Acid to Prevent Bone Loss in the First Year after Kidney Transplantation
- PMID: 30606784
- PMCID: PMC6362629
- DOI: 10.1681/ASN.2018060656
A Randomized Trial of Zoledronic Acid to Prevent Bone Loss in the First Year after Kidney Transplantation
Abstract
Background: Bone and mineral disorders commonly affect kidney transplant (KTx) recipients and have been associated with a high risk of fracture. Bisphosphonates may prevent or treat bone loss in such patients, but there is concern that these drugs might induce adynamic bone disease (ABD).
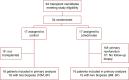
Methods: In an open label, randomized trial to assess the safety and efficacy of zoledronate for preventing bone loss in the first year after kidney transplant, we randomized 34 patients before transplant to receive zoledronate or no treatment. We used dual-energy x-ray absorptiometry (DXA), high-resolution peripheral quantitative computed tomography (HR-pQCT), and bone biopsies to evaluate changes in bone in the 32 evaluable participants between the time of KTx and 12 months post-transplant.
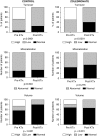
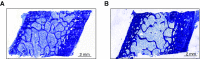
Results: Both groups of patients experienced decreased bone turnover after KTx, but zoledronate itself did not affect this outcome. Unlike previous studies, DXA showed no post-transplant bone loss in either group; we instead observed an increase of bone mineral density in both lumbar spine and total hip sites, with a significant positive effect of zoledronate. However, bone biopsies showed post-transplant impairment of trabecular connectivity (and no benefit from zoledronate); HR-pQCT detected trabecular bone loss at the peripheral skeleton, which zoledronate partially attenuated.
Conclusions: Current immunosuppressive regimens do not contribute to post-transplant central skeleton trabecular bone loss, and zoledronate does not induce ABD. Because fractures in transplant recipients are most commonly peripheral fractures, clinicians should consider bisphosphonate use in patients at high fracture risk who have evidence of significantly low bone mass at these sites at the time of KTx.
Keywords: bone biopsy; kidney disease; kidney transplantation; mineral metabolism; renal osteodystrophy; zoledronic acid.
Copyright © 2019 by the American Society of Nephrology.
Figures

Comment in
-
Biphosphonate Therapy, Risk of Fracture, and Sites of Bone Mineral Density Assessments in Kidney Transplantation.J Am Soc Nephrol. 2019 May;30(5):905. doi: 10.1681/ASN.2019010079. Epub 2019 Mar 25. J Am Soc Nephrol. 2019. PMID: 30910935 Free PMC article. No abstract available.
-
Authors' Reply.J Am Soc Nephrol. 2019 May;30(5):906. doi: 10.1681/ASN.2019020124. Epub 2019 Mar 25. J Am Soc Nephrol. 2019. PMID: 30910936 Free PMC article. No abstract available.
References
-
- Rojas E, Carlini RG, Clesca P, Arminio A, Suniaga O, De Elguezabal K, et al. .: The pathogenesis of osteodystrophy after renal transplantation as detected by early alterations in bone remodeling. Kidney Int 63: 1915–1923, 2003 - PubMed
-
- Neves CL, dos Reis LM, Batista DG, Custodio MR, Graciolli FG, Martin RC, et al. .: Persistence of bone and mineral disorders 2 years after successful kidney transplantation. Transplantation 96: 290–296, 2013 - PubMed
-
- Brandenburg VM, Politt D, Ketteler M, Fassbender WJ, Heussen N, Westenfeld R, et al. .: Early rapid loss followed by long-term consolidation characterizes the development of lumbar bone mineral density after kidney transplantation. Transplantation 77: 1566–1571, 2004 - PubMed
-
- Bonani M, Frey D, Brockmann J, Fehr T, Mueller TF, Saleh L, et al. .: Effect of twice-yearly denosumab on prevention of bone mineral density loss in de novo kidney transplant recipients: A randomized controlled trial. Am J Transplant 16: 1882–1891, 2016 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

