Efficacy of first-line treatments for multiple myeloma patients not eligible for stem cell transplantation: a network meta-analysis
- PMID: 30606791
- PMCID: PMC6518894
- DOI: 10.3324/haematol.2018.206912
Efficacy of first-line treatments for multiple myeloma patients not eligible for stem cell transplantation: a network meta-analysis
Abstract
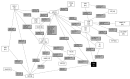
Decision making for patients with multiple myeloma (MM) not transplant eligible (NTE) is complicated by a lack of head-to-head comparisons of standards of care, the increase in the choice of treatment modalities, and the promising results that are rapidly evolving from studies with novel regimens. To support evidence-based decision making, we performed a network meta-analysis for NTE MM patients that synthesizes direct and indirect evidence and enables a comparison of all treatments. Relevant randomized clinical trials were identified by a systematic literature review in EMBASE®, MEDLINE®, MEDLINE®-in-Process and the Cochrane Central Register of Controlled Trials for January 1999 to March 2016. Efficacy outcomes [i.e. the hazard ratio (HR) and 95% confidence interval (95%CI) for progression-free survival] were extracted and synthesized in a random effects network-meta analysis. In total, 24 studies were identified including 21 treatments. According to the network-meta analysis, the HR for progression-free survival was favorable for all NTE MM treatments compared to dexamethasone (HR: 0.19-0.90). Daratumumab-bortezomib-melphalan-prednisone and bortezomib-melphalan-prednisone-thalidomide with bortezomib-thalidomide maintenance were identified as the most effective treatments (HR: 0.19, 95%CI: 0.08-0.45 and HR: 0.22, 95%CI: 0.10-0.51, respectively). HR and 95%CI for currently recommended treatments, bortezomib-lenalidomide-dexamethasone, bortezomib-melphalan-prednisone, and lenalidomide-dexamethasone compared to dexamethasone, were 0.31 (0.16-0.59), 0.39 (0.20-0.75), and 0.44 (0.29-0.65), respectively. In addition to identifying the most effective treatment options, we illustrate the additional value and evidence of network meta-analysis in clinical practice. In the current treatment landscape, the results of network meta-analysis may support evidence-based decisions and ultimately help to optimize treatment and outcomes of NTE MM patients.
Copyright© 2019 Ferrata Storti Foundation.
Figures


References
-
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. - PubMed
-
- Mateos MV, San Miguel JF. How should we treat newly diagnosed multiple myeloma patients? Hematology Am Soc Hematol Educ Program. 2013;2013:488–495. - PubMed
-
- Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-updagger. Ann Oncol. 2017; 128(suppl_4):iv52–iv61. - PubMed
-
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371(10):906–917. - PubMed
-
- San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

