Biosynthesis of mycobacterial methylmannose polysaccharides requires a unique 1- O-methyltransferase specific for 3- O-methylated mannosides
- PMID: 30606802
- PMCID: PMC6338870
- DOI: 10.1073/pnas.1813450116
Biosynthesis of mycobacterial methylmannose polysaccharides requires a unique 1- O-methyltransferase specific for 3- O-methylated mannosides
Abstract
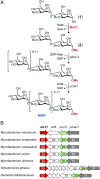
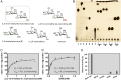
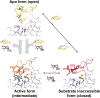
Mycobacteria are a wide group of organisms that includes strict pathogens, such as Mycobacterium tuberculosis, as well as environmental species known as nontuberculous mycobacteria (NTM), some of which-namely Mycobacterium avium-are important opportunistic pathogens. In addition to a distinctive cell envelope mediating critical interactions with the host immune system and largely responsible for their formidable resistance to antimicrobials, mycobacteria synthesize rare intracellular polymethylated polysaccharides implicated in the modulation of fatty acid metabolism, thus critical players in cell envelope assembly. These are the 6-O-methylglucose lipopolysaccharides (MGLP) ubiquitously detected across the Mycobacterium genus, and the 3-O-methylmannose polysaccharides (MMP) identified only in NTM. The polymethylated nature of these polysaccharides renders the intervening methyltransferases essential for their optimal function. Although the knowledge of MGLP biogenesis is greater than that of MMP biosynthesis, the methyltransferases of both pathways remain uncharacterized. Here, we report the identification and characterization of a unique S-adenosyl-l-methionine-dependent sugar 1-O-methyltransferase (MeT1) from Mycobacterium hassiacum that specifically blocks the 1-OH position of 3,3'-di-O-methyl-4α-mannobiose, a probable early precursor of MMP, which we chemically synthesized. The high-resolution 3D structure of MeT1 in complex with its exhausted cofactor, S-adenosyl-l-homocysteine, together with mutagenesis studies and molecular docking simulations, unveiled the enzyme's reaction mechanism. The functional and structural properties of this unique sugar methyltransferase further our knowledge of MMP biosynthesis and provide important tools to dissect the role of MMP in NTM physiology and resilience.
Keywords: 3D structure; Mycobacterium; S-adenosyl-l-methionine; polymethylated polysaccharides; sugar methyltransferase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Falkinham JO., 3rd Environmental sources of nontuberculous mycobacteria. Clin Chest Med. 2015;36:35–41. - PubMed
-
- Gray GR, Ballou CE. Isolation and characterization of a polysaccharide containing 3-O-methyl-D-mannose from Mycobacterium phlei. J Biol Chem. 1971;246:6835–6842. - PubMed
-
- Mendes V, Maranha A, Alarico S, Empadinhas N. Biosynthesis of mycobacterial methylglucose lipopolysaccharides. Nat Prod Rep. 2012;29:834–844. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

