Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field
- PMID: 30606819
- PMCID: PMC7745124
- DOI: 10.1126/science.aat9077
Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field
Erratum in
-
Erratum for the Research Article "Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field" by P. F. South, A. P. Cavanagh, H. W. Liu, D. R. Ort.Science. 2019 Aug 2;365(6452):eaay8818. doi: 10.1126/science.aay8818. Science. 2019. PMID: 31371580 No abstract available.
Abstract
Photorespiration is required in C3 plants to metabolize toxic glycolate formed when ribulose-1,5-bisphosphate carboxylase-oxygenase oxygenates rather than carboxylates ribulose-1,5-bisphosphate. Depending on growing temperatures, photorespiration can reduce yields by 20 to 50% in C3 crops. Inspired by earlier work, we installed into tobacco chloroplasts synthetic glycolate metabolic pathways that are thought to be more efficient than the native pathway. Flux through the synthetic pathways was maximized by inhibiting glycolate export from the chloroplast. The synthetic pathways tested improved photosynthetic quantum yield by 20%. Numerous homozygous transgenic lines increased biomass productivity by >40% in replicated field trials. These results show that engineering alternative glycolate metabolic pathways into crop chloroplasts while inhibiting glycolate export into the native pathway can drive increases in C3 crop yield under agricultural field conditions.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution License 4.0 (CC BY).
Conflict of interest statement
The authors declare no competing interests
Figures


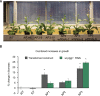



Comment in
-
Improving crop yield.Science. 2019 Jan 4;363(6422):32-33. doi: 10.1126/science.aav8979. Science. 2019. PMID: 30606834 No abstract available.
References
-
- Zhu X. G, Long S. P, Ort D. R, Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 61, 235–261 (2010). - PubMed
-
- Ainsworth E. A, Long S. P, What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 165, 351–371 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

