Real-world benefit of nivolumab in a Canadian non-small-cell lung cancer cohort
- PMID: 30607113
- PMCID: PMC6291291
- DOI: 10.3747/co.25.4287
Real-world benefit of nivolumab in a Canadian non-small-cell lung cancer cohort
Abstract
Background: Nivolumab was the first immuno-oncology agent available for the treatment of lung cancer in Canada. In the present study, we evaluated the real-world benefit of nivolumab in Canadian patients with lung cancer.
Methods: Patients included in the cohort were identified from a registry of patients treated through expanded access to nivolumab before and after Health Canada approval. Demographics were collected from the application forms. Outcome data for the duration of treatment and survival were collected retrospectively.
Results: In contrast to the randomized clinical trial populations, our study cohort included patients who were older (median age: 66 years; range: 36-92 years) and who had an Eastern Cooperative Oncology Group performance status of 2 (8.9%). Despite the poorer-prognosis cohort, median overall survival was 12.0 months, which is comparable to the survival demonstrated in the randomized phase iii trials of nivolumab in lung cancer. Median time to treatment discontinuation was 3.45 months and was similar for all patient subgroups, including poorer-prognosis groups such as those with a performance status of 2, those 75 years of age and older, and those with brain metastases.
Conclusions: Nivolumab given in a real-world clinical setting was associated with results similar to those reported in the phase iii clinical trial setting.
Keywords: Nivolumab; duration of treatment; non-small-cell lung cancer; overall survival; real-world data.
Conflict of interest statement
CONFLICT OF INTEREST DISCLOSURES We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: CL reports personal fees from Bristol–Myers Squibb, Merck, Pfizer, and AstraZeneca, outside the submitted work; CB reports personal fees from Bristol–Myers Squibb, Merck, and AstraZeneca, outside the submitted work; MNR reports personal fees from Bristol–Myers Squibb outside the submitted work; FAS reports personal fees and a grant from Bristol–Myers Squibb; personal fees, stock ownership, and an advisory role with Eli Lilly; personal fees, stock ownership, and an advisory role with Astra-Zeneca; personal fees from Roche/Genentech; personal fees from Merck Sharp and Dohme; personal fees from Merck Serono; and grants from Pfizer, AstraZeneca/MedImmune, Roche Canada, and Merrimack, outside the submitted work; JR reports personal fees from Bristol–Myers Squibb, Roche, Merck, AstraZeneca, Boehringer Ingelheim, and Pfizer, outside the submitted work; SO reports personal fees from Bristol–Myers Squibb outside the submitted work; RAJ reports grants, personal fees, and an advisory role with Bristol-Myers Squibb; grants, personal fees, and an advisory role with Merck Sharp and Dohme; personal fees and an advisory role with Roche Canada; personal fees and an advisory role with Boehringer Ingelheim; grants and personal fees from AstraZeneca/MedImmune; personal fees from Eli Lilly; personal fees and an advisory role with Pfizer; personal fees from Amgen; personal fees and an advisory role with Novartis, outside the submitted work; FP, FR, and JV report being employees of Bristol–Myers Squibb. The remaining authors have no conflicts to disclose.
Figures
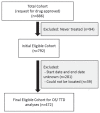
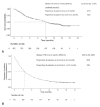
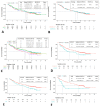

References
-
- Canadian Agency for Drugs and Technologies in Health (cadth), pan-Canadian Oncology Drug Review. Opdivo for Non-Small Cell Lung Cancer—Details [Web page] Ottawa, ON: CADTH; 2016. [Available at: https://www.cadth.ca/opdivo-non-small-cell-lung-cancer-details; cited 1 February 2018]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

