Use of Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy in Patients with Axillary Node-Positive Breast Cancer in Diagnosis
- PMID: 30607165
- PMCID: PMC6310714
- DOI: 10.4048/jbc.2018.21.e54
Use of Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy in Patients with Axillary Node-Positive Breast Cancer in Diagnosis
Abstract
Purpose: This study aimed to evaluate the effects of sentinel lymph node biopsy (SLNB) on recurrence and survival after neoadjuvant chemotherapy (NAC) in breast cancer patients with cytology-proven axillary node metastasis.
Methods: We selected patients who were diagnosed with invasive breast cancer and axillary lymph node metastasis and were treated with NAC followed by curative surgery between January 2007 and December 2014. We classified patients into three groups: group A, negative sentinel lymph node (SLN) status and no further dissection; group B, negative SLN status with backup axillary lymph node dissection (ALND); and group C, no residual axillary metastasis on pathology with standard ALND.
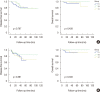
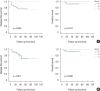
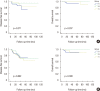
Results: The median follow-up time was 51 months (range, 3-122 months) and the median number of retrieved SLNs was 5 (range, 2-9). The SLN identification rate was 98.3% (234/238 patients), and the false negative rate of SLNB after NAC was 7.5%. There was no significant difference in axillary recurrence-free survival (p=0.118), disease-free survival (DFS; p=0.578) or overall survival (OS; p=0.149) among groups A, B, and C. In the subgroup analysis of breast pathologic complete response (pCR) status, there was no significant difference in DFS (p=0.271, p=0.892) or OS (p=0.207, p=0.300) in the breast pCR and non-pCR patients.
Conclusion: These results suggest that SLNB can be feasible and oncologically safe after NAC for cytology-determined axillary node metastasis patients and could help reduce arm morbidity and lymphedema by avoiding ALND in SLN-negative patients.
Keywords: Breast neoplasms; Lymph node excision; Neoadjuvant therapy; Sentinel lymph node biopsy.
Conflict of interest statement
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
Figures


References
-
- Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–2493. - PubMed
-
- Vlastos G, Mirza NQ, Lenert JT, Hunt KK, Ames FC, Feig BW, et al. The feasibility of minimally invasive surgery for stage IIA, IIB, and IIIA breast carcinoma patients after tumor downstaging with induction chemotherapy. Cancer. 2000;88:1417–1424. - PubMed
-
- Park S, Lee JE, Paik HJ, Ryu JM, Bae SY, Lee SK, et al. Feasibility and prognostic effect of sentinel lymph node biopsy after neoadjuvant chemotherapy in cytology-proven, node-positive breast cancer. Clin Breast Cancer. 2017;17:e19–e29. - PubMed
-
- Enokido K, Watanabe C, Nakamura S, Ogiya A, Osako T, Akiyama F, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with an initial diagnosis of cytology-proven lymph node-positive breast cancer. Clin Breast Cancer. 2016;16:299–304. - PubMed
LinkOut - more resources
Full Text Sources

