High throughput screening against pantothenate synthetase identifies amide inhibitors against Mycobacterium tuberculosis and Staphylococcus aureus
- PMID: 30607322
- PMCID: PMC6314788
- DOI: 10.1007/s40203-018-0046-4
High throughput screening against pantothenate synthetase identifies amide inhibitors against Mycobacterium tuberculosis and Staphylococcus aureus
Abstract
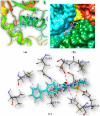
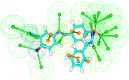
Abstract: Pantothenate is a crucial enzyme for the synthesis of coenzyme A and acyl carrier protein in Mycobacterium tuberculosis and Staphylococcus aureus. It is indispensable for the growth and survival of these bacteria. Amides analogs are designed and have been used as inhibitors of pantothenate synthetase. Molecular docking approach has been used to design and predict the drug activity of molecule to the specific disease. In this work, more than hundred amides have been screened by Discovery Studio molecular docking programme to search best suitable molecule for the treatment of Mycobacterium tuberculosis. Pharmacophore generation has been done to recognize the binding modes of inhibitors in the receptor active site. To observe the stability and flexibility of inhibitors molecular dynamics (MD) simulation has been done; Lipinski's rule of five protocols is followed to screen drug likeness and ADMET (absorption, distribution, metabolism, excretion and toxicity) filtration is also used to value toxicity. DFT computation of optimized geometry and derivation of MOs has been used to correlate the drug likeness. The small difference in energy between HOMO and LUMO may help to activate the drug in the protein environment quickly. 2-Hydroxy-5-[(E)-2-{4-[(prop-2-enamido)sulfonyl]phenyl}diazen-1-yl]benzoic acid (M1) shows best theoretical efficiency against Mycobacterium tuberculosis (MTB) pantothenate synthetase and so does 2-hydroxy-5-[(E)-2-{4-[(2-phenylacetamido)sulfonyl]phenyl}diazen-1-yl]benzoic acid (M2) against Staphylococcus aureus pantothenate synthetase. These compounds also bind to Adenine-Thymine region of tuberculosis DNA.
Keywords: ADMET; Heterocyclic amide compounds; MD simulation; Molecular docking; Pantothenate synthetase inhibitors; Structure based drug design.
Figures








Similar articles
-
Inhibitors of pantothenate synthetase of Mycobacterium tuberculosis - a medicinal chemist perspective.RSC Adv. 2020 Oct 7;10(61):37098-37115. doi: 10.1039/d0ra07398a. eCollection 2020 Oct 7. RSC Adv. 2020. PMID: 35521286 Free PMC article. Review.
-
Sulfonamide derivatives as Mycobacterium tuberculosis inhibitors: in silico approach.In Silico Pharmacol. 2018 Mar 22;6(1):4. doi: 10.1007/s40203-018-0041-9. eCollection 2018. In Silico Pharmacol. 2018. PMID: 30607317 Free PMC article.
-
Lead identification against Mycobacterium tuberculosis using highly enriched active molecules against pantothenate synthetase.J Biomol Struct Dyn. 2024;42(20):11080-11097. doi: 10.1080/07391102.2023.2260483. Epub 2023 Sep 25. J Biomol Struct Dyn. 2024. PMID: 37747063
-
Identification of the Seaweed Metabolites as Potential Anti-tubercular Agents Against Human Pantothenate synthetase: An In Silico Approach.Curr Microbiol. 2023 Aug 14;80(10):318. doi: 10.1007/s00284-023-03422-w. Curr Microbiol. 2023. PMID: 37578562
-
5-tert-butyl-N-pyrazol-4-yl-4,5,6,7-tetrahydrobenzo[d]isoxazole-3-carboxamide derivatives as novel potent inhibitors of Mycobacterium tuberculosis pantothenate synthetase: initiating a quest for new antitubercular drugs.J Med Chem. 2008 Apr 10;51(7):1999-2002. doi: 10.1021/jm701372r. Epub 2008 Mar 13. J Med Chem. 2008. PMID: 18335974 Review.
Cited by
-
Identification of Putative Vaccine and Drug Targets against the Methicillin-Resistant Staphylococcus aureus by Reverse Vaccinology and Subtractive Genomics Approaches.Molecules. 2022 Mar 24;27(7):2083. doi: 10.3390/molecules27072083. Molecules. 2022. PMID: 35408485 Free PMC article.
-
Inhibitors of pantothenate synthetase of Mycobacterium tuberculosis - a medicinal chemist perspective.RSC Adv. 2020 Oct 7;10(61):37098-37115. doi: 10.1039/d0ra07398a. eCollection 2020 Oct 7. RSC Adv. 2020. PMID: 35521286 Free PMC article. Review.
-
Screening of Compounds for Anti-tuberculosis Activity, and in vitro and in vivo Evaluation of Potential Candidates.Front Microbiol. 2021 Jun 30;12:658637. doi: 10.3389/fmicb.2021.658637. eCollection 2021. Front Microbiol. 2021. PMID: 34276592 Free PMC article.
-
Vitamin in the Crosshairs: Targeting Pantothenate and Coenzyme A Biosynthesis for New Antituberculosis Agents.Front Cell Infect Microbiol. 2020 Dec 15;10:605662. doi: 10.3389/fcimb.2020.605662. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33384970 Free PMC article. Review.
-
Exploring the Potential of Phytocannabinoids Against Multidrug-Resistant Bacteria.Plants (Basel). 2025 Jun 20;14(13):1901. doi: 10.3390/plants14131901. Plants (Basel). 2025. PMID: 40647911 Free PMC article.
References
-
- Bartzatt R, Cirillo SL, Cirillo JD. Sulfonamide agents for treatment of Staphylococcus MRSA and MSSA infections of the central nervous system. Cent Nerv Syst Agents Med Chem. 2010;10(1):84–90. - PubMed
-
- Beard DA, Qian H. Chemical biophysics: quantitative analysis of cellular systems. Cambridge: Cambridge University Press; 2010.
-
- Becke AD. Density-functional thermochemistry. The role of exact exchange. J Chem Phys. 1993;98(7):5648–5652.
-
- Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5. New York: W. H. Freeman; 2002.
LinkOut - more resources
Full Text Sources

