Implementing a quality management system using good clinical laboratory practice guidelines at KEMRI-CMR to support medical research
- PMID: 30607370
- PMCID: PMC6305232
- DOI: 10.12688/wellcomeopenres.14860.2
Implementing a quality management system using good clinical laboratory practice guidelines at KEMRI-CMR to support medical research
Abstract
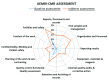
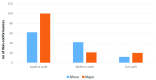
Background: Good Clinical Laboratory Practice (GCLP) is a standard that helps ensure the quality and reliability of research data through principles of Good Laboratory Practice (GLP) and Good Clinical Practice (GCP). The implementation of GCLP includes careful documentation of procedures, competencies and safety measures. Implementation of GCLP is influenced by existing resources and quality systems, thus laboratories in low- and middle-income countries may face additional challenges. Methods: This paper describes implementation of GCLP at the Kenya Medical Research Institute-Center for Microbiology Research (KEMRI-CMR) as part of a quality system to support medical research. This study employed assessment, twinning (institutional mentorship) model, conducting relevant training workshops and Kaizen 5S approaches to implement an effective quality management system using GCLP standard. This was achieved through a collaboration between the KEMRI/Wellcome Trust Research Programme (KWTRP) and KEMRI-CMR. The aim was compliance and continuous monitoring to meet international GCLP standards in a way that could be replicated in other research organizations. Results: Following a baseline assessment in March 2017, training, mentorship and a cycle of quality audit and corrective action using a Kaizen 5S approach (sorting, setting in order, shining, standardizing and sustaining) was established. Laboratory personnel were trained in writing standard operating procedures and analytical plans, microbiological techniques, and good documentation practice. Mid-term and exit assessments demonstrated significant declines in non-conformances across all GCLP elements. KEMRI-CMR achieved GCLP accreditation in May 2018 by Qualogy Ltd (UK). Conclusions: Involving all the laboratory personnel in implementation of quality management system processes is critical to success. An institutional mentorship (twinning) approach shows potential for future collaborations between accredited and non-accredited organizations to accelerate the implementation of high-quality management systems and continuous improvement.
Keywords: Good Clinical Laboratory Practice; Quality Assurance; Quality system; medical research; quality management system..
Conflict of interest statement
No competing interests were disclosed.
Figures


References
-
- Gumba H: KEMRI-CMR Data collected & analyzed.xls. figshare Dataset.2018.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

