The use of Toxoplasma gondii tachyzoites produced in HeLa cells adhered to Cytodex 1 microcarriers as antigen in serological assays: an application of microcarrier technology
- PMID: 30607647
- PMCID: PMC6368493
- DOI: 10.1007/s10616-018-0269-6
The use of Toxoplasma gondii tachyzoites produced in HeLa cells adhered to Cytodex 1 microcarriers as antigen in serological assays: an application of microcarrier technology
Abstract
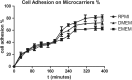
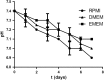
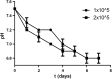
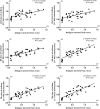
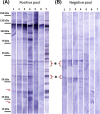
Toxoplasma gondii can infect nearly all warm-blooded animals, including humans. In the laboratory diagnosis of toxoplasmosis, serological tests have importance in detecting antibody response. Traditionally T. gondii tachyzoites grown in vivo are being used as an antigen source in serological assays. Currently, tachyzoites produced in vitro are being tested as an antigen source in order to decrease animal use. Microcarrier technology allowed us to grow anchorage-dependent host cells on microcarrier suspension in short time and approximately 10 times more than traditional flask technique. The ability of T. gondii tachyzoites to grow in host cells adhered to microcarriers has not been analyzed yet. In this study, we aimed to develop a novel in vitro culture method to produce T. gondii tachyzoites abundantly using HeLa cells adhered to Cytodex 1 microcarriers. Initially, the growth of HeLa cells adhered to Cytodex 1 was analyzed using RPMI 1640, DMEM, and EMEM. Next, HeLa cells with a concentration of 1 × 105 cells/ml and 2 × 105 cells/ml were adhered to Cytodex 1 and grown in spinner flasks. Then, T. gondii tachyzoites were inoculated with 1:1 and 2:1 cell:tachyzoite ratios to HeLa cells adhered to microcarriers in spinner flaks. During continuous production in spinner flasks, tachyzoites were harvested at the 2nd, 4th, and 7th day of culture and the quality of antigens produced from these tachyzoites were tested in ELISA and Western Blotting using sera of patients with toxoplasmosis. The optimization studies showed that finest HeLa inoculation value was 2 × 105 cells/ml using RPMI 1640, and the cell:tachyzoite ratio to obtain the highest tachyzoite yield (17.1 × 107) was 1:1 at the 4th day of inoculation. According to the results of ELISA comparing HeLa cell and mouse derived antigens, the highest correlation with mouse antigen was achieved at the 4th day of HeLa cell culture with 1:1 HeLa:tachyzoite ratio (P < 0.0001). The sensitivity and specificity ratios of ELISA were 100%. In addition, Western blotting banding patterns of the antigen derived at the 4th day of HeLa cell culture with 1:1 HeLa:tachyzoite ratio was comparable with mouse derived antigen. Overall, this novel methodology can be an alternative source of antigen in diagnostic assays, decrease animal use for antigen production, and contribute to the solution of ethical and economic problems.
Keywords: Antigen; HeLa cell; Microcarriers; Toxoplasma gondii.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
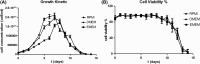
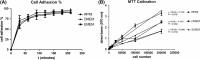


Similar articles
-
Toxoplasma gondii RH Ankara: production of evolving tachyzoites using a novel cell culture method.Exp Parasitol. 2011 May;128(1):1-8. doi: 10.1016/j.exppara.2011.01.019. Epub 2011 Feb 4. Exp Parasitol. 2011. PMID: 21296077
-
Behaviour of Toxoplasma gondii RH Ankara strain tachyzoites during continuous production in various cell lines.Parasitology. 2006 Mar;132(Pt 3):315-9. doi: 10.1017/S0031182005009078. Epub 2005 Dec 1. Parasitology. 2006. PMID: 16318650
-
Cell-culture system for continuous production of Toxoplasma gondii tachyzoites.Eur J Clin Microbiol Infect Dis. 1999 Dec;18(12):879-84. doi: 10.1007/s100960050423. Eur J Clin Microbiol Infect Dis. 1999. PMID: 10691199
-
[Problems and limitations of conventional and innovative methods for the diagnosis of Toxoplasmosis in humans and animals].Parassitologia. 2004 Jun;46(1-2):177-81. Parassitologia. 2004. PMID: 15305712 Review. Italian.
-
[Study and application of surface antigen in tachyzoites of Toxoplasma gondii].Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2014 Dec;26(6):687-9. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2014. PMID: 25856903 Review. Chinese.
Cited by
-
Cell Immortality: In Vitro Effective Techniques to Achieve and Investigate Its Applications and Challenges.Life (Basel). 2024 Mar 21;14(3):417. doi: 10.3390/life14030417. Life (Basel). 2024. PMID: 38541741 Free PMC article. Review.
-
The Truman Show for protozoan parasites: A review of in vitro cultivation platforms.PLoS Negl Trop Dis. 2021 Aug 26;15(8):e0009668. doi: 10.1371/journal.pntd.0009668. eCollection 2021 Aug. PLoS Negl Trop Dis. 2021. PMID: 34437538 Free PMC article. Review.
References
-
- Blaker GJ, Birch JR, Pirt SJ. The glucose, insulin and glutamine requirements of suspension cultures of HeLa cells in a defined culture medium. J Cell Sci. 1971;9:529–537. - PubMed
LinkOut - more resources
Full Text Sources

