Diagnostic and prognostic biomarkers for HAND
- PMID: 30607890
- PMCID: PMC6610810
- DOI: 10.1007/s13365-018-0705-6
Diagnostic and prognostic biomarkers for HAND
Abstract
In 2007, the nosology for HIV-1-associated neurocognitive disorders (HAND) was updated to a primarily neurocognitive disorder. However, currently available diagnostic tools lack the sensitivity and specificity needed for an accurate diagnosis for HAND. Scientists and clinicians, therefore, have been on a quest for an innovative biomarker to diagnose (i.e., diagnostic biomarker) and/or predict (i.e., prognostic biomarker) the progression of HAND in the post-combination antiretroviral therapy (cART) era. The present review examined the utility and challenges of four proposed biomarkers, including neurofilament light (NFL) chain concentration, amyloid (i.e., sAPPα, sAPPβ, amyloid β) and tau proteins (i.e., total tau, phosphorylated tau), resting-state functional magnetic resonance imaging (fMRI), and prepulse inhibition (PPI). Although significant genotypic differences have been observed in NFL chain concentration, sAPPα, sAPPβ, amyloid β, total tau, phosphorylated tau, and resting-state fMRI, inconsistencies and/or assessment limitations (e.g., invasive procedures, lack of disease specificity, cost) challenge their utility as a diagnostic and/or prognostic biomarker for milder forms of neurocognitive impairment (NCI) in the post-cART era. However, critical evaluation of the literature supports the utility of PPI as a powerful diagnostic biomarker with high accuracy (i.e., 86.7-97.1%), sensitivity (i.e., 89.3-100%), and specificity (i.e., 79.5-94.1%). Additionally, the inclusion of multiple CSF and/or plasma markers, rather than a single protein, may provide a more sensitive diagnostic biomarker for HAND; however, a pressing need for additional research remains. Most notably, PPI may serve as a prognostic biomarker for milder forms of NCI, evidenced by its ability to predict later NCI in higher-order cognitive domains with regression coefficients (i.e., r) greater than 0.8. Thus, PPI heralds an opportunity for the development of a brief, noninvasive diagnostic and promising prognostic biomarker for milder forms of NCI in the post-cART era.
Keywords: Diagnostic biomarker; HIV-1-associated neurocognitive disorders; Prepulse inhibition; Prognostic biomarker.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Figures
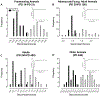
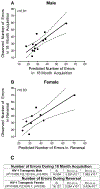
Similar articles
-
Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease.J Neurovirol. 2019 Oct;25(5):702-709. doi: 10.1007/s13365-018-0695-4. Epub 2019 Jan 4. J Neurovirol. 2019. PMID: 30610738 Free PMC article. Review.
-
Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection.PLoS One. 2014 Dec 26;9(12):e116081. doi: 10.1371/journal.pone.0116081. eCollection 2014. PLoS One. 2014. PMID: 25541953 Free PMC article.
-
Neurofilament light chain in blood is negatively associated with neuropsychological performance in HIV-infected adults and declines with initiation of antiretroviral therapy.J Neurovirol. 2018 Dec;24(6):695-701. doi: 10.1007/s13365-018-0664-y. Epub 2018 Aug 13. J Neurovirol. 2018. PMID: 30105502 Free PMC article.
-
Aging, comorbidities, and the importance of finding biomarkers for HIV-associated neurocognitive disorders.J Neurovirol. 2019 Oct;25(5):673-685. doi: 10.1007/s13365-019-00735-0. Epub 2019 Mar 13. J Neurovirol. 2019. PMID: 30868422 Free PMC article. Review.
-
Association Between Longitudinal Plasma Neurofilament Light and Neurodegeneration in Patients With Alzheimer Disease.JAMA Neurol. 2019 Jul 1;76(7):791-799. doi: 10.1001/jamaneurol.2019.0765. JAMA Neurol. 2019. PMID: 31009028 Free PMC article.
Cited by
-
NRF2 Antagonizes HIV-1 Tat and Methamphetamine-Induced BV2 Cell Ferroptosis by Regulating SLC7A11.Neurotox Res. 2023 Oct;41(5):398-407. doi: 10.1007/s12640-023-00645-4. Epub 2023 Apr 15. Neurotox Res. 2023. PMID: 37060393
-
Prospective Neuropsychological and Plasma Biomarker Changes in Treatment-Naïve People Living with HIV After Antiretroviral Treatment Initiation.Biomedicines. 2025 Jul 12;13(7):1704. doi: 10.3390/biomedicines13071704. Biomedicines. 2025. PMID: 40722775 Free PMC article.
-
HIV-Associated Apathy/Depression and Neurocognitive Impairments Reflect Persistent Dopamine Deficits.Cells. 2021 Aug 21;10(8):2158. doi: 10.3390/cells10082158. Cells. 2021. PMID: 34440928 Free PMC article. Review.
-
Cognitive impairment in people living with HIV: consensus recommendations for a new approach.Nat Rev Neurol. 2023 Jul;19(7):424-433. doi: 10.1038/s41582-023-00813-2. Epub 2023 Jun 13. Nat Rev Neurol. 2023. PMID: 37311873 Review.
-
Role of Autophagy in HIV-1 and Drug Abuse-Mediated Neuroinflammaging.Viruses. 2022 Dec 23;15(1):44. doi: 10.3390/v15010044. Viruses. 2022. PMID: 36680084 Free PMC article. Review.
References
-
- Anderson AM, Easley KA, Kasher N, Franklin D, Heaton RK, Zetterberg H, Blennow K, Gisslén M, Letendre SL (2018) Neurofilament light chain in blood is negatively associated with neuropsychological performance in HIV-infected adults and declines with initiation of antiretroviral therapy. J Neurovirol [Epub ahead of print]. doi: 10.1007/s13365-018-0664-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

