Faster Hypothermia Induced by Esophageal Cooling Improves Early Markers of Cardiac and Neurological Injury After Cardiac Arrest in Swine
- PMID: 30608213
- PMCID: PMC6404192
- DOI: 10.1161/JAHA.118.010283
Faster Hypothermia Induced by Esophageal Cooling Improves Early Markers of Cardiac and Neurological Injury After Cardiac Arrest in Swine
Abstract
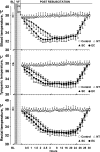
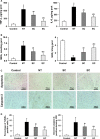
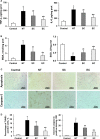
Background After cardiopulmonary resuscitation, the protective effects of therapeutic hypothermia induced by conventional cooling are limited. Recently, esophageal cooling ( EC ) has been shown to be an effective, easily performed approach to induce therapeutic hypothermia. In this study we investigated the efficacy of EC and its effects on early markers of postresuscitation cardiac and neurological injury in a porcine model of cardiac arrest. Methods and Results Thirty-two male domestic swine were randomized into 4 groups: sham control, normothermia, surface cooling, and EC . Sham animals underwent the surgical preparation only. Ventricular fibrillation was induced and untreated for 8 minutes while defibrillation was attempted after 5 minutes of cardiopulmonary resuscitation. At 5 minutes after resuscitation, therapeutic hypothermia was induced by either EC or surface cooling to reach a target temperature of 33°C until 24 hours postresuscitation, followed by a rewarming rate of 1°C/h for 5 hours. The temperature was normally maintained in the control and normothermia groups. After resuscitation, a significantly faster decrease in blood temperature was observed in the EC group than in the surface cooling group (2.8±0.7°C/h versus 1.5±0.4°C/h; P<0.05). During the maintenance and rewarming phases the temperature was maintained at an even level between the 2 groups. Postresuscitation cardiac and neurological damage was significantly improved in the 2 hypothermic groups compared with the normothermia group; however, the protective effects were significantly greater in the EC group. Conclusions In a porcine model of cardiac arrest, faster hypothermia successfully induced by EC was significantly better than conventional cooling in improving early markers of postresuscitation cardiac and neurological injury.
Keywords: cardiac arrest; cardiopulmonary resuscitation; esophageal cooling; organ protection; therapeutic hypothermia.
Figures



References
-
- Laurent I, Monchi M, Chiche JD, Joly LM, Spaulding C, Bourgeois B, Cariou A, Rozenberg A, Carli P, Weber S, Dhainaut JF. Reversible myocardial dysfunction in survivors of out‐of‐hospital cardiac arrest. J Am Coll Cardiol. 2002;40:2110–2116. - PubMed
-
- Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126–2128. - PubMed
-
- Lu X, Ma L, Sun S, Xu J, Zhu C, Tang W. The effects of the rate of postresuscitation rewarming following hypothermia on outcomes of cardiopulmonary resuscitation in a rat model. Crit Care Med. 2014;42:e106–e113. - PubMed
-
- Chenoune M, Lidouren F, Adam C, Pons S, Darbera L, Bruneval P, Ghaleh B, Zini R, Dubois‐Randé JL, Carli P, Vivien B, Ricard JD, Berdeaux A, Tissier R. Ultrafast and whole‐body cooling with total liquid ventilation induces favorable neurological and cardiac outcomes after cardiac arrest in rabbits. Circulation. 2011;124:901–911, 1–7. - PMC - PubMed
-
- Lin S, Scales DC, Dorian P, Kiss A, Common MR, Brooks SC, Goodman SG, Salciccioli JD, Morrison LJ. Targeted temperature management processes and outcomes after out‐of‐hospital cardiac arrest: an observational cohort study. Crit Care Med. 2014;42:2565–2574. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

