Meta-analysis of overall incidence and risk of ALK inhibitors-induced liver toxicities in advanced non-small-cell lung cancer
- PMID: 30608384
- PMCID: PMC6344205
- DOI: 10.1097/MD.0000000000013726
Meta-analysis of overall incidence and risk of ALK inhibitors-induced liver toxicities in advanced non-small-cell lung cancer
Abstract
Aim: Activation of the anaplastic lymphoma kinase (ALK) gene has been found in several human cancers, including non-small-cell lung cancer (NSCLC). Currently, novel drugs targeting ALK gene have been extensively investigated in NSCLC. However, concerns about ALK inhibitors-induced liver toxicities have been increasing.
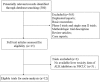
Materials and methods: Eligible prospective clinical studies have been searched in several databases. Primary outcomes of interest were incidence rates of liver toxicities, relative risks (RRs), and 95% confidence intervals (CIs).
Results: Data from 2418 patients (1873 in the experimental arm; 545 in the control arm) were included. The incidences of all-grade alanine transaminase (ALT) and aspartate aminotransferase (AST) elevation were 26.0% (95% CI: 17.4%-37%), and 23.2% (95% CI, 16.7%-31.4%), respectively. The incidences of high-grade ALT and AST elevation were 8.4% (95% CI, 5.1%-13.4% and 7.0% (95% CI: 5.4%-9.0%), respectively. Sub-group analysis according to the ALK inhibitors found that pooled incidence of liver toxicities associated with ceritinib was higher than that of crizotinib and alectinib. In comparison with chemotherapy, ALK inhibitors significantly increased the all-grade and high-grade ALT elevation (RR 2.37, 95% CI, 1.97-2.86; P < .001; RR 7.34, 95% CI, 3.95-13.63; P < .001) and AST elevation (RR 3.27, 95% CI, 2.47-4.34; P < .001; RR 11.54, 95% CI, 4.33-30.7; P < .001), respectively. No publication bias was detected for RR of ALT and AST.
Conclusions: The findings of the present study offer substantial evidence that ALK inhibitors treatment in advanced NSCLC significantly increases the risk of developing all-grade and high-grade liver toxicities in comparison with controls. Clinicians should recognize liver toxicities promptly as early interventions may alleviate future complications.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures




Similar articles
-
Efficacy and Safety of ALK Tyrosine Kinase Inhibitors in Elderly Patients with Advanced ALK-Positive Non-Small Cell Lung Cancer: Findings from the Real-Life Cohort.Oncol Res Treat. 2019;42(5):275-282. doi: 10.1159/000499086. Epub 2019 Apr 5. Oncol Res Treat. 2019. PMID: 30955009
-
A retrospective study of alectinib versus ceritinib in patients with advanced non-small-cell lung cancer of anaplastic lymphoma kinase fusion in whom crizotinib treatment failed.BMC Cancer. 2021 Mar 24;21(1):309. doi: 10.1186/s12885-021-08005-1. BMC Cancer. 2021. PMID: 33761908 Free PMC article.
-
Incidence and risk of hepatic toxicities associated with anaplastic lymphoma kinase inhibitors in the treatment of non-small-cell lung cancer: a systematic review and meta-analysis.Oncotarget. 2017 Dec 16;9(10):9480-9488. doi: 10.18632/oncotarget.23840. eCollection 2018 Feb 6. Oncotarget. 2017. PMID: 29507704 Free PMC article.
-
Effect of alectinib versus crizotinib on progression-free survival, central nervous system efficacy and adverse events in ALK-positive non-small cell lung cancer: a systematic review and meta-analysis.Ann Palliat Med. 2020 Jul;9(4):1782-1796. doi: 10.21037/apm-19-643. Epub 2020 Jun 8. Ann Palliat Med. 2020. PMID: 32527124
-
Alectinib for advanced ALK-positive non-small-cell lung cancer.Am J Health Syst Pharm. 2018 Apr 15;75(8):515-522. doi: 10.2146/ajhp170266. Epub 2018 Feb 21. Am J Health Syst Pharm. 2018. PMID: 29467147 Review.
Cited by
-
Hydrogen therapy can be used to control tumor progression and alleviate the adverse events of medications in patients with advanced non-small cell lung cancer.Med Gas Res. 2020 Apr-Jun;10(2):75-80. doi: 10.4103/2045-9912.285560. Med Gas Res. 2020. PMID: 32541132 Free PMC article. Clinical Trial.
-
Safety Profile and Hepatotoxicity of Anaplastic Lymphoma Kinase Tyrosine Kinase Inhibitors: A Disproportionality Analysis Based on FDA Adverse Event Reporting System Database.Toxics. 2025 Mar 14;13(3):210. doi: 10.3390/toxics13030210. Toxics. 2025. PMID: 40137538 Free PMC article.
-
New Target Therapies in Advanced Non-Small Cell Lung Cancer: A Review of the Literature and Future Perspectives.J Clin Med. 2020 Nov 3;9(11):3543. doi: 10.3390/jcm9113543. J Clin Med. 2020. PMID: 33153004 Free PMC article. Review.
-
Successful Treatment with Lorlatinib after the Development of Alectinib-Induced Liver Damage in ALK-Positive Non-Small-Cell Lung Cancer: A Case Report.Case Rep Oncol. 2021 Mar 1;14(1):197-201. doi: 10.1159/000513624. eCollection 2021 Jan-Apr. Case Rep Oncol. 2021. PMID: 33776703 Free PMC article.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10–29. - PubMed
-
- Iacono D, Chiari R, Metro G, et al. Future options for ALK-positive non-small cell lung cancer. Lung Cancer 2015;87:211–9. - PubMed
-
- Peters S, Taron M, Bubendorf L, et al. Treatment and detection of ALK-rearranged NSCLC. Lung Cancer 2013;81:145–54. - PubMed
-
- Blakely C, Jahan T. Emerging antiangiogenic therapies for non-small-cell lung cancer. Expert Rev Anticancer Ther 2011;11:1607–18. - PubMed
-
- Maione P, Rossi A, Bareschino MA, et al. Factors driving the choice of the best second-line treatment of advanced NSCLC. Rev Recent Clin Trials 2011;6:44–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

