Identification of Genes Involved in Lipid Biosynthesis through de novo Transcriptome Assembly from Cocos nucifera Developing Endosperm
- PMID: 30608545
- PMCID: PMC6498750
- DOI: 10.1093/pcp/pcy247
Identification of Genes Involved in Lipid Biosynthesis through de novo Transcriptome Assembly from Cocos nucifera Developing Endosperm
Abstract
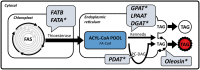
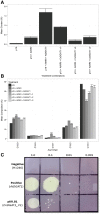
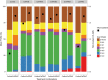
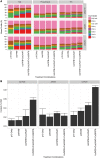
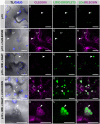
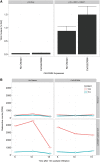
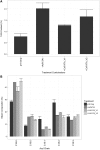
Cocos nucifera (coconut), a member of the Arecaceae family, is an economically important woody palm that is widely grown in tropical and subtropical regions. The coconut palm is well known for its ability to accumulate large amounts of oil, approximately 63% of the seed weight. Coconut oil varies significantly from other vegetable oils as it contains a high proportion of medium-chain fatty acids (MCFA; 85%). The unique composition of coconut oil raises interest in understanding how the coconut palm produces oil of a high saturated MCFA content, and if such an oil profile could be replicated via biotechnology interventions. Although some gene discovery work has been performed there is still a significant gap in the knowledge associated with coconut's oil production pathways. In this study, a de novo transcriptome was assembled for developing coconut endosperm to identify genes involved in the synthesis of lipids, particularly triacylglycerol. Of particular interest were thioesterases, acyltransferases and oleosins because of their involvement in the processes of releasing fatty acids for assembly, esterification of fatty acids into glycerolipids and protecting oils from degradation, respectively. It is hypothesized that some of these genes may exhibit a strong substrate preference for MCFA and hence may assist the future development of vegetable oils with an enriched MCFA composition. In this study, we identified and confirmed functionality of five candidate genes from the gene families of interest. This study will benefit future work in areas of increasing vegetable oil production and the tailoring of oil fatty acid compositions.
Keywords: De novo assembly; Nicotiana; Coconut; DGAT; Endosperm; FATA; Fatty acid; GPAT9; Medium chain; Oleosin; PDAT; Thioesterase; Transcriptome; Triacylglycerol.
� The Author(s) 2019. Published by Oxford University Press on behalf of Japanese Society of Plant Physiologists.
Figures

Similar articles
-
A reconfigured Kennedy pathway which promotes efficient accumulation of medium-chain fatty acids in leaf oils.Plant Biotechnol J. 2017 Nov;15(11):1397-1408. doi: 10.1111/pbi.12724. Epub 2017 May 3. Plant Biotechnol J. 2017. PMID: 28301719 Free PMC article.
-
Genetic control of fatty acid composition in coconut (Cocos nucifera), African oil palm (Elaeis guineensis), and date palm (Phoenix dactylifera).Planta. 2019 Feb;249(2):333-350. doi: 10.1007/s00425-018-3003-x. Epub 2018 Sep 7. Planta. 2019. PMID: 30194535
-
Comparative transcriptome analysis of three oil palm fruit and seed tissues that differ in oil content and fatty acid composition.Plant Physiol. 2013 Jul;162(3):1337-58. doi: 10.1104/pp.113.220525. Epub 2013 Jun 4. Plant Physiol. 2013. PMID: 23735505 Free PMC article.
-
Variety of Plant Oils: Species-Specific Lipid Biosynthesis.Plant Cell Physiol. 2024 Jun 27;65(6):845-862. doi: 10.1093/pcp/pcad147. Plant Cell Physiol. 2024. PMID: 37971406 Review.
-
Coconut Milk and Coconut Oil: Their Manufacture Associated with Protein Functionality.J Food Sci. 2018 Aug;83(8):2019-2027. doi: 10.1111/1750-3841.14223. Epub 2018 Jul 13. J Food Sci. 2018. PMID: 30004125 Review.
Cited by
-
Integration of miRNAs, Degradome, and Transcriptome Omics Uncovers a Complex Regulatory Network and Provides Insights Into Lipid and Fatty Acid Synthesis During Sesame Seed Development.Front Plant Sci. 2021 Jul 29;12:709197. doi: 10.3389/fpls.2021.709197. eCollection 2021. Front Plant Sci. 2021. PMID: 34394165 Free PMC article.
-
Comprehensive Analysis of Metabolome and Transcriptome Reveals the Regulatory Network of Coconut Nutrients.Metabolites. 2023 May 24;13(6):683. doi: 10.3390/metabo13060683. Metabolites. 2023. PMID: 37367842 Free PMC article.
-
Is CRISPR/Cas9 a way forward to fast-track genetic improvement in commercial palms? Prospects and limits.Front Plant Sci. 2022 Dec 12;13:1042828. doi: 10.3389/fpls.2022.1042828. eCollection 2022. Front Plant Sci. 2022. PMID: 36578341 Free PMC article. Review.
-
Upregulated Lipid Biosynthesis at the Expense of Starch Production in Potato (Solanum tuberosum) Vegetative Tissues via Simultaneous Downregulation of ADP-Glucose Pyrophosphorylase and Sugar Dependent1 Expressions.Front Plant Sci. 2019 Nov 12;10:1444. doi: 10.3389/fpls.2019.01444. eCollection 2019. Front Plant Sci. 2019. PMID: 31781148 Free PMC article.
-
Tracing Key Molecular Regulators of Lipid Biosynthesis in Tuber Development of Cyperus esculentus Using Transcriptomics and Lipidomics Profiling.Genes (Basel). 2021 Sep 24;12(10):1492. doi: 10.3390/genes12101492. Genes (Basel). 2021. PMID: 34680888 Free PMC article.
References
-
- Arkcoll D. (1988) Lauric oil resources. Econ. Bot. 42: 195–205.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

