Colonization factors among enterotoxigenic Escherichia coli isolates from children with moderate-to-severe diarrhea and from matched controls in the Global Enteric Multicenter Study (GEMS)
- PMID: 30608930
- PMCID: PMC6343939
- DOI: 10.1371/journal.pntd.0007037
Colonization factors among enterotoxigenic Escherichia coli isolates from children with moderate-to-severe diarrhea and from matched controls in the Global Enteric Multicenter Study (GEMS)
Abstract
Background: Enterotoxigenic Escherichia coli (ETEC) encoding heat-stable enterotoxin (ST) alone or with heat-labile enterotoxin (LT) cause moderate-to-severe diarrhea (MSD) in developing country children. The Global Enteric Multicenter Study (GEMS) identified ETEC encoding ST among the top four enteropathogens. Since the GEMS objective was to provide evidence to guide development and implementation of enteric vaccines and other interventions to diminish diarrheal disease morbidity and mortality, we examined colonization factor (CF) prevalence among ETEC isolates from children age <5 years with MSD and from matched controls in four African and three Asian sites. We also assessed strength of association of specific CFs with MSD.
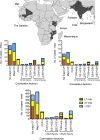
Methodology/principal findings: MSD cases enrolled at healthcare facilities over three years and matched controls were tested in a standardized manner for many enteropathogens. To identify ETEC, three E. coli colonies per child were tested by polymerase chain reaction (PCR) to detect genes encoding LT, ST; confirmed ETEC were examined by PCR for major CFs (Colonization Factor Antigen I [CFA/I] or Coli Surface [CS] antigens CS1-CS6) and minor CFs (CS7, CS12, CS13, CS14, CS17, CS18, CS19, CS20, CS21, CS30). ETEC from 806 cases had a single toxin/CF profile in three tested strains per child. Major CFs, components of multiple ETEC vaccine candidates, were detected in 66.0% of LT/ST and ST-only cases and were associated with MSD versus matched controls by conditional logistic regression (p≤0.006); major CFs detected in only 25.0% of LT-only cases weren't associated with MSD. ETEC encoding exclusively CS14, identified among 19.9% of 291 ST-only and 1.5% of 259 LT/ST strains, were associated with MSD (p = 0.0011). No other minor CF exhibited prevalence ≥5% and significant association with MSD.
Conclusions/significance: Major CF-based efficacious ETEC vaccines could potentially prevent up to 66% of pediatric MSD cases due to ST-encoding ETEC in developing countries; adding CS14 extends coverage to ~77%.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests. We note that the following authors are involved in the development of vaccines to prevent diarrhea caused by enterotoxigenic Escherichia coli: James P. Nataro: Has been named in patents for technology that may have relevance for ETEC vaccine technology; JPN is also a co-investigator on grants from non-profit agencies that support ETEC vaccine development. Halvor Sommerfelt: Has been named in patents for technology that may have relevance for ETEC vaccine technology including: INT Application Number PCT/IB2014/000267; INT Publication Number WO2014128555 A2 “ETEC Vaccine”. European Patent Application No. 14711297.3 United States Patent Application No. 14/769,342. China Patent Application No. 201480022329.6. Puntervoll P, Sommerfelt H, Clements J, Nataro JP, Zhang W, Taxt A. HS is also a co-investigator on grants from non-profit agencies that support ETEC vaccine development Ann-Mari Svennerholm: Has shares in the University of Göteborg spin-out biotech company Gotovax AB which is entitled to royalty from Scandinavian Biopharma on sales in travelers of the ETEC vaccine Etvax if it becomes a commercial product. A-MS is a co-inventor on ETEC vaccine patent application owned by Scandinavian Biopharma and has a research grant from the Swedish Foundation for Strategic Research for infection biology research. Myron M. Levine: Is a co-inventor of patents for ETEC vaccine technology including US patent #6,902,736 B2. “Isolation and characterization of the CSA operon (ETEC-CS4 pili) and methods of using same.” He is a member of the Scientific Advisory Board of the PaxVax Corporation. MML is also the recipient for grants relevant to ETEC vaccine development including the Enteric Center of Excellence for Translational Research (Enteric-CETR) “Immunoprophylactic Strategies to Control Emerging Enteric Infections” (NIAID U19AI109776; MML, PI)
Figures


References
-
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013; 382(9888):209–22. 10.1016/S0140-6736(13)60844-2 - DOI - PubMed
-
- Levine MM. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987; 155:377–89. - PubMed

