Glycosaminoglycans from Alzheimer's disease hippocampus have altered capacities to bind and regulate growth factors activities and to bind tau
- PMID: 30608949
- PMCID: PMC6319808
- DOI: 10.1371/journal.pone.0209573
Glycosaminoglycans from Alzheimer's disease hippocampus have altered capacities to bind and regulate growth factors activities and to bind tau
Abstract
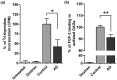
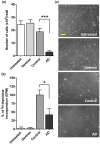
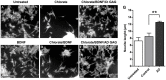
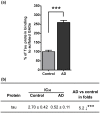
Glycosaminoglycans (GAGs), including heparan sulfates and chondroitin sulfates, are major components of the extracellular matrix. Upon interacting with heparin binding growth factors (HBGF), GAGs participate to the maintaintenance of tissue homeostasis and contribute to self-healing. Although several processes regulated by HBGF are altered in Alzheimer's disease, it is unknown whether the brain GAG capacities to bind and regulate the function of HBGF or of other heparin binding proteins, as tau, are modified in this disease. Here, we show that total sulfated GAGs from hippocampus of Alzheimer's disease have altered capacities to bind and potentiate the activities of growth factors including FGF-2, VEGF, and BDNF while their capacity to bind to tau is remarkable increased. Alterations of GAG structures and capacities to interact with and regulate the activity of heparin binding proteins might contribute to impaired tissue homeostasis in the Alzheimer's disease brain.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Bottaro DP. The role of extracellular matrix heparan sulfate glycosaminoglycan in the activation of growth factor signaling pathways. Ann N Y Acad Sci. 2002; 961:158 . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

