High-fat diet accelerates extreme obesity with hyperphagia in female heterozygous Mecp2-null mice
- PMID: 30608967
- PMCID: PMC6319720
- DOI: 10.1371/journal.pone.0210184
High-fat diet accelerates extreme obesity with hyperphagia in female heterozygous Mecp2-null mice
Abstract
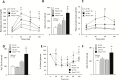
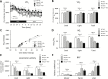
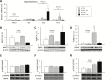
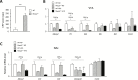
Rett syndrome (RTT) is an X-linked neurodevelopmental disorder caused by mutation of the methyl-CpG-binding protein 2 (MECP2) gene. Although RTT has been associated with obesity, the underlying mechanism has not yet been elucidated. In this study, female heterozygous Mecp2-null mice (Mecp2+/- mice), a model of RTT, were fed a normal chow diet or high-fat diet (HFD), and the changes in molecular signaling pathways were investigated. Specifically, we examined the expression of genes related to the hypothalamus and dopamine reward circuitry, which represent a central network of feeding behavior control. In particular, dopamine reward circuitry has been shown to regulate hedonic feeding behavior, and its disruption is associated with HFD-related changes in palatability. The Mecp2+/- mice that were fed the normal chow showed normal body weight and food consumption, whereas those fed the HFD showed extreme obesity with hyperphagia, an increase of body fat mass, glucose intolerance, and insulin resistance compared with wild-type mice fed the HFD (WT-HFD mice). The main cause of obesity in Mecp2+/--HFD mice was a remarkable increase in calorie intake, with no difference in oxygen consumption or locomotor activity. Agouti-related peptide mRNA and protein levels were increased, whereas proopiomelanocortin mRNA and protein levels were reduced in Mecp2+/--HFD mice with hyperleptinemia, which play an essential role in appetite and satiety in the hypothalamus. The conditioned place preference test revealed that Mecp2+/- mice preferred the HFD. Tyrosine hydroxylase and dopamine transporter mRNA levels in the ventral tegmental area, and dopamine receptor and dopamine- and cAMP-regulated phosphoprotein mRNA levels in the nucleus accumbens were significantly lower in Mecp2+/--HFD mice than those of WT-HFD mice. Thus, HFD feeding induced dysregulation of food intake in the hypothalamus and dopamine reward circuitry, and accelerated the development of extreme obesity associated with addiction-like eating behavior in Mecp2+/- mice.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Kleefstra T, Yntema HG, Oudakker AR, Romein T, Sistermans E, Nillessen W, et al. De novo MeCP2 frameshift mutation in a boy with moderate mental retardation, obesity and gynaecomstia. Clin Genet. 2002; 61: 359–362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

