In vitro anti-inflammatory effects of AZD8999, a novel bifunctional muscarinic acetylcholine receptor antagonist /β2-adrenoceptor agonist (MABA) compound in neutrophils from COPD patients
- PMID: 30608978
- PMCID: PMC6319735
- DOI: 10.1371/journal.pone.0210188
In vitro anti-inflammatory effects of AZD8999, a novel bifunctional muscarinic acetylcholine receptor antagonist /β2-adrenoceptor agonist (MABA) compound in neutrophils from COPD patients
Abstract
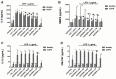
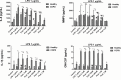
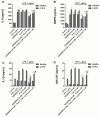
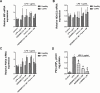
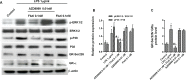
Recent evidence indicates that AZD8999 (LAS190792), a novel muscarinic acetylcholine receptor antagonist and β2-adrenoceptor agonist (MABA) in development for chronic respiratory diseases, induces potent and sustained relaxant effects in human bronchi by adressing both muscarinic acetylcholine receptors and β2-adrenoceptor. However, the anti-inflammatory effects of the AZD8999 monotherapy or in combination with corticosteroids are unknown. This study investigates the anti-inflammatory effects of AZD8999 in monotherapy and combined with fluticasone propionate in neutrophils from healthy and chronic obstructive pulmonary disease (COPD) patients. Peripheral blood neutrophils from healthy and COPD patients were incubated with AZD8999 and fluticasone propionate, individually or in combination, for 1h followed by lipopolysaccharide (LPS) stimulation for 6h. The IL-8, MMP9, IL-1β, and GM-CSF release was measured in cell culture supernatants. AZD8999 shows ~ 50% maximum inhibitory effect and similar potency inhibiting the released cytokines in neutrophils from healthy and COPD patients. However, while fluticasone propionate suppresses mediator release in neutrophils from healthy patients, COPD neutrophils are less sensitive. The combination of non-effective concentrations of AZD8999 (0.01nM) with non-effective concentrations of fluticasone propionate (0.1nM) shows synergistic anti-inflammatory effects. The studied mechanisms that may be involved in the synergistic anti-inflammatory effects of this combination include the increase of glucocorticoid receptor (GR)α and MKP1 expression, the induction of glucocorticoid response element (GRE) activation and the decrease of ERK1/2, P38 and GR-Ser226 phosphorylations compared with monotherapies. In summary, AZD8999 shows anti-inflammatory effects in neutrophils from COPD patients and induces synergistic anti-inflammatory effects when combined with fluticasone propionate, supporting the use of MABA/ICS combination therapy in COPD.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: Marta Calbet, Mònica Aparici and Montserrat Miralpeix are employees of Almirall, the company that discovered the MABA mollecule AZD8999 analyzed in this work". This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
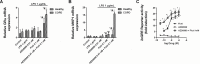
References
-
- Gedebjerg A, Szepligeti SK, Wackerhausen LH, Horvath-Puho E, Dahl R, Hansen JG, et al. Prediction of mortality in patients with chronic obstructive pulmonary disease with the new Global Initiative for Chronic Obstructive Lung Disease 2017 classification: a cohort study. The Lancet Respiratory medicine. 2018;6(3):204–12. 10.1016/S2213-2600(18)30002-X - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

