Iron for the treatment of restless legs syndrome
- PMID: 30609006
- PMCID: PMC6353229
- DOI: 10.1002/14651858.CD007834.pub3
Iron for the treatment of restless legs syndrome
Abstract
Background: Restless legs syndrome (RLS) is a common neurologic disorder that is associated with peripheral iron deficiency in a subgroup of patients. It is unclear whether iron therapy is effective treatment for RLS.
Objectives: To evaluate the efficacy and safety of oral or parenteral iron for the treatment of restless legs syndrome (RLS) when compared with placebo or other therapies.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, PsycNFO, and CINAHL for the time period January 1995 to September 2017. We searched reference lists for additional published studies. We searched Clinicaltrials.gov and other clinical trial registries (September 2017) for ongoing or unpublished studies.
Selection criteria: Controlled trials comparing any formulation of iron with placebo, other medications, or no treatment, in adults diagnosed with RLS according to expert clinical interview or explicit diagnostic criteria.
Data collection and analysis: Two review authors independently extracted data and assessed trial quality, with discussion to reach consensus in the case of any disagreement. The primary outcome considered in this review was restlessness or unpleasant sensations, as experienced subjectively by the patient. We combined treatment/control differences in the outcomes across studies using random-effects meta-analyses. We analysed continuous data using mean differences (MDs) where possible and performed standardised mean difference (SMD) analyses when different measurements were used across studies. We calculated risk ratios (RRs) for dichotomous data using the Mantel-Haenszel method and 95% confidence intervals (CIs). We analysed study heterogeneity using the I2 statistic. We used standard methodological procedures expected by Cochrane. We performed GRADE analysis using GRADEpro.
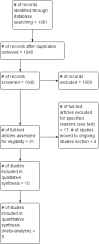
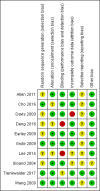
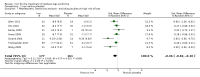
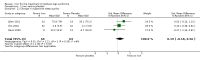
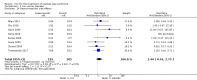
Main results: We identified and included 10 studies (428 total participants, followed for 2-16 weeks) in this review. Our primary outcome was restlessness or uncomfortable leg sensations, which was quantified using the International Restless Legs Scale (IRLS) (range, 0 to 40) in eight trials and a different RLS symptom scale in a ninth trial. Nine studies compared iron to placebo and one study compared iron to a dopamine agonist (pramipexole). The possibility for bias among the trials was variable. Three studies had a single element with high risk of bias, which was lack of blinding in two and incomplete outcome data in one. All studies had at least one feature resulting in unclear risk of bias.Combining data from the seven trials using the IRLS to compare iron and placebo, use of iron resulted in greater improvement in IRLS scores (MD -3.78, 95% CI -6.25 to -1.31; I2= 66%, 7 studies, 345 participants) measured 2 to 12 weeks after treatment. Including an eighth study, which measured restlessness using a different scale, use of iron remained beneficial compared to placebo (SMD -0.74, 95% CI -1.26 to -0.23; I2 = 80%, 8 studies, 370 participants). The GRADE assessment of certainty for this outcome was moderate.The single study comparing iron to a dopamine agonist (pramipexole) found a similar reduction in RLS severity in the two groups (MD -0.40, 95% CI -5.93 to 5.13, 30 participants).Assessment of secondary outcomes was limited by small numbers of trials assessing each outcome. Iron did not improve quality of life as a dichotomous measure (RR 2.01, 95% CI 0.54 to 7.45; I2=54%, 2 studies, 39 participants), but did improve quality of life measured on continuous scales (SMD 0.51, 95% CI 0.15 to 0.87; I2= 0%, 3 studies, 128 participants), compared to placebo. Subjective sleep quality was no different between iron and placebo groups (SMD 0.19, 95% CI -0.18 to 0.56; I2 = 9%, 3 studies, 128 participants), nor was objective sleep quality, as measured by change in sleep efficiency in a single study (-35.5 +/- 92.0 versus -41.4 +/- 98.2, 18 participants). Periodic limb movements of sleep were not significantly reduced with iron compared to placebo ( SMD -0.19, 95% CI -0.70 to 0.32; I2 = 0%, 2 studies, 60 participants). Iron did not improve sleepiness compared to placebo, as measured on the Epworth Sleepiness Scale (data not provided, 1 study, 60 participants) but did improve the daytime tiredness item of the RLS-6 compared to placebo (least squares mean difference -1.5, 95% CI -2.5 to -0.6; 1 study, 110 participants). The GRADE rating for secondary outcomes ranged from low to very low.Prespecified subgroup analyses showed more improvement with iron in those trials studying participants on dialysis. The use of low serum ferritin levels as an inclusion criteria and the use or oral versus intravenous iron did not show significant subgroup differences.Iron did not result in significantly more adverse events than placebo (RR 1.48, 95% CI 0.97 to 2.25; I2=45%, 6 studies, 298 participants). A single study reported that people treated with iron therapy experienced fewer adverse events than the active comparator pramipexole.
Authors' conclusions: Iron therapy probably improves restlessness and RLS severity in comparison to placebo. Iron therapy may not increase the risk of side effects in comparison to placebo. We are uncertain whether iron therapy improves quality of life in comparison to placebo. Iron therapy may make little or no difference to pramipexole in restlessness and RLS severity, as well as in the risk of adverse events. The effect on secondary outcomes such as quality of life, daytime functioning, and sleep quality, the optimal timing and formulation of administration, and patient characteristics predicting response require additional study.
Conflict of interest statement
Dr. Trotti reports no declarations of interest. Dr. Becker reports no declarations of interest.
Figures














Update of
-
Iron for restless legs syndrome.Cochrane Database Syst Rev. 2012 May 16;5(5):CD007834. doi: 10.1002/14651858.CD007834.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2019 Jan 04;1:CD007834. doi: 10.1002/14651858.CD007834.pub3. PMID: 22592724 Free PMC article. Updated.
References
References to studies included in this review
Allen 2011 {published and unpublished data}
-
- Allen RP, Adler CH, Du W, Butcher A, Bregman DB, Earley CJ. Clinical efficacy and safety of IV ferric carboxymaltose (FCM) treatment of RLS: a multi‐centred, placebo‐controlled preliminary clinical trial. Sleep Medicine 2011;12(9):906‐13. - PubMed
Cho 2016 {published data only}
-
- Cho YW, Allen RP, Earley CJ. Clinical efficacy of ferric carboxymaltose treatment in patients with restless legs syndrome. Sleep Medicine 2016;25:16‐23. - PubMed
Davis 2000 {published data only}
-
- Davis BJ, Rajput A, Rajput ML, Aul EA, Eichhorn GR. A randomized, double‐blind, placebo‐controlled trial of iron in restless legs syndrome. European Neurology 2000;43:70‐5. - PubMed
Deng 2016 {published data only (unpublished sought but not used)}
Earley 2009 {published data only (unpublished sought but not used)}
Grote 2009 {published and unpublished data}
-
- Grote L, Leissner L, Hedner J, Ulfberg J. A randomized, double‐blind, placebo‐controlled, multi‐center study of intravenous iron sucrose and placebo in the treatment of restless legs syndrome. Movement Disorders 2009;24(10):1445‐52. - PubMed
Lee 2014 {published data only}
-
- Lee CS, Lee SD, Kang SH, Park HY, Yoon IY. Comparison of the efficacies of oral iron and pramipexole for the treatment of restless legs syndromre patients with low serum ferritin. European Journal of Neurology 2014;21:260‐6. - PubMed
Sloand 2004 {published data only (unpublished sought but not used)}
-
- Sloand JA, Shelly MA, Feigin A, Bernstein P, Monk RD. A double‐blind, placebo‐controlled trial of intravenous iron dextran therapy in patients with ESRD and restless legs syndrome. American Journal of Kidney Diseases 2004;43(4):663‐70. - PubMed
Trenkwalder 2017 {published and unpublished data}
Wang 2009 {published data only (unpublished sought but not used)}
-
- Wang J, O'Reilly B, Venkataraman R, Mysliwiec V, Mysliwiec A. Efficacy of oral iron in patients with restless legs syndrome and a low‐normal ferritin: A randomized, double‐blind, placebo‐controlled study. Sleep Medicine 2009;10(9):973‐5. - PubMed
References to studies excluded from this review
Auerbach 2014 {published data only}
-
- Auerbach M. The role of intravenous iron in the treatment of restless legs. American Journal of Hematology 2014;89(10):1016. - PubMed
Birgegard 2010 {published data only}
-
- Birgegard G, Schneider K, Ulfberg J. High incidence of iron depletion and restless legs syndrome (RLS) in regular blood donors: intravenous iron sucrose substitution more effective than oral iron. Vox Sang 2010;99(4):354‐61. - PubMed
Cho 2011 {published data only}
-
- Cho Y, Kim D, Shin W, Earley CJ, Allen RP. Lower molecular weight intravenous iron dextran for restless legs syndrome. Sleep 2011;34 Suppl 1:A204. - PubMed
Earley 2004 {published data only}
-
- Earley CJ, Heckler D, Allen RP. The treatment of restless legs syndrome with intravenous iron dextran. Sleep Medicine 2004;5:231‐5. - PubMed
Earley 2005 {published data only}
-
- Earley CJ, Hecker D, Allen RP. Repeated IV doses of iron provides effective supplemental treatment of restless legs syndrome. Sleep Medicine 2005;6:301‐5. - PubMed
Ekermo 2013 {published data only}
-
- Ekermo BE, Forsberg P, Schedvin G, Berlin G. An open, randomised, clinical study of oral versus intravenous iron for iron substitution in blood donors. Transfusion 2013;53(Suppl 2):36A.
Halterman 2007 {published data only}
-
- Halterman MW, Sloand JA. An update on the use of intravenous iron for the treatment of restless legs syndrome in end stage renal disease. Salud (i) Ciencia 2007;15:860‐5.
Konofal 2008 {published data only}
-
- Konofal E, Lecendreux M, Deron J, Marchand M, Cortese S, Zaim M, et al. Effects of iron supplementation on attention deficit hyperactivity disorder in children. Pediatric Neurology 2008;38:20‐6. - PubMed
Macher 2015 {published and unpublished data}
-
- Macher S, Drexler C, Stojakovic T, Lindenau I, Schrock M, Reinprecht C, et al. Intravenous high‐dose iron supplementation for blood donors with iron deficiency. Haematologica 2015;100(Suppl 1):140.
Mohri 2008 {published data only}
-
- Mohri I, Kato‐Nishimura K, Tachibana N, Ozono K, Taniike M. Restless legs syndrome (RLS): an unrecognized cause for bedtime problems and insomnia in children. Sleep Medicine 2008;9:701‐2. - PubMed
NCT00895232 {published data only}
-
- NCT00895232. Iron Sucrose In The Treatment of Restless Legs Syndrome: Safety of Three Dose Regimens as Evaluated by Clinical Assessments. clinicaltrials.gov/ct2/show/NCT00895232 (first received 5/7/09).
O'Keeffe 1994 {published data only}
-
- O'Keeffe ST, Gavin K, Lavan JN. Iron status and restless legs syndrome in the elderly. Age and Aging 1994;23:200‐3. - PubMed
Ondo 2010 {published data only}
-
- Ondo WG. Intravenous iron dextran for severe refractory restless legs syndrome. Sleep Medicine 2010;11(5):494‐6. - PubMed
Simakajornboon 2003 {published data only}
-
- Simakajornboon N, Gozal D, Vlasic V, Mack C, Sharon D, McGinley BM. Periodic limb movements in sleep and iron status in children. Sleep 2003;26(6):735‐8. - PubMed
Vishwakarma 2013 {published and unpublished data}
-
- Vishwakarma K, Kalra J, Gupta R, Sharma M, Sharma T. A double‐blind, randomized, controlled trial to compare the efficacy and tolerability of fixed doses of ropinirole, bupropion, and iron in treatment of restless legs syndrome (Willis‐Ekbom disease). Annals of Indian Academy of Neurology 2016;19(4):472‐7. - PMC - PubMed
Zhang 2014 {published data only}
-
- Zhang X, Chen WW, Huang WJ. Efficacy of the low‐dose Saccharum iron treatment of idiopathic restless legs syndrome. Panminerva Medica 2015;57(3):109‐13. - PubMed
Zilberman 2010 {published data only}
-
- Zilberman M, Silverberg DS, Schwartz D, Oksenberg A. Restless legs syndrome (RLS) in anemic patients with congestive heart failure and chronic renal failure: lack of effect of anemia treatment. International Journal of Cardiology 2010;143(2):205‐7. - PubMed
References to ongoing studies
ISRCTN01222004 {unpublished data only}
-
- ISRCTN01222004. Iron supplementation in iron deficiency. http://www.isrctn.com/ISRCTN01222004 (date applied 10/22/2009).
NCT00685815 {unpublished data only}
-
- NCT00685815. Intravenous Iron Metabolism in Restless Legs Syndrome. https://clinicaltrials.gov/ct2/show/NCT00685815 (first submitted 5/23/08).
NCT01895231 {unpublished data only}
-
- NCT01895231. A Study of Intravenous Iron Isomaltoside 1000 (Monofer®) Administered by Infusions to Iron‐deficient Blood Donors. https://clinicaltrials.gov/ct2/show/NCT01895231 (first submitted 6/26/13).
NCT02826681 {unpublished data only}
-
- NCT02826681. Randomized, Placebo‐controlled Trial of Ferric Carboxymaltose in RLS Patients With Iron‐deficiency Anemia. https://clinicaltrials.gov/ct2/show/NCT02826681 (first submitted 6/30/16).
Additional references
Abetz 2005
-
- Abetz L, Vallow SM, Kirsch J, Allen RP, Washburn T, Earley CJ. Validation of the Restless Legs Syndrome Quality of Life questionnaire. Value Health 2005;8(2):157‐67. - PubMed
Allen 2003
-
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Medicine 2003;4:101‐19. - PubMed
Allen 2004
-
- Allen R. Dopamine and iron in the pathophysiology of restless legs syndrome. Sleep Medicine 2004;5:385‐91. - PubMed
Allen 2013
-
- Allen RP, Auerbach S, Bahrain H, Auerbach M, Earley CJ. The prevalence and impact of restless legs syndrome on patients with iron deficiency anemia. American Journal of Hematology 2013;88:261‐4. - PubMed
Allen 2013b
-
- Allen RP. Minimal clinically significant change for the International Restless Legs Syndrome Study Group rating scale in clinical trials is a score of 3. Sleep Medicine 2013;14(11):1229. - PubMed
Allen 2014
-
- Allen RP, Picchietti DL, Garcia‐Borreguero D, Ondo WG, Walters AS, Winkelman JW, et al. International Restless Legs Syndrome Study Group. Restless legs syndrome/Willis‐Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria‐history, rationale, description, and significance. Sleep Medicine 2014;15(8):860‐73. - PubMed
Aurora 2012
Avni 2015
-
- Avni T, Bieber A, Grossman A, Green H, Leibovici L, Gafter‐Gvili A. The safety of intravenous iron preparations: systematic review and meta‐analysis. Mayo Clinic Proceedings 2015;90(1):12‐23. - PubMed
Berger 2004
-
- Berger K, Luedemann J, Trenkwalder C, John U, Kessler C. Sex and the risk of restless legs syndrome in the general population. Archives of Internal Medicine 2004;164:196‐202. - PubMed
Bliwise 2006
Buysse 1989
-
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research.. Psychiatry Research 1989;28(2):193‐213. - PubMed
Connor 2003
-
- Connor JR, Boyer PJ, Menzies SL, Dellinger B, Allen RP, Ondo WG, et al. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology 2003;61:304‐9. - PubMed
Earley 2000
-
- Earley CJ, Allen RP, Beard JL, Connor JR. Insight into the pathophysiology of restless legs syndrome. Journal of Neuroscience Research 2000;62:623‐8. - PubMed
Earley 2014
-
- Earley CJ, Connor J, Garcia‐Borreguero D, Jenner P, Winkelman J, Zee PC, et al. Altered brain iron homeostasis and dopaminergic function in Restless Legs Syndrome (Willis‐Ekbom Disease). Sleep Medicine 2014;15(11):1288‐301. - PubMed
Garcia‐Borreguero 2012
-
- Garcia‐Borreguero D, Ferini‐Strambi L, Kohnen R, O'Keeffe S, Trenkwalder C, Hogl B, et al. European guidelines on management of restless legs syndrome: report of a joint task force by the European Federation of Neurological Societies, the European Neurological Society, and the European Sleep Research Society. European Journal of Neurology 2012;19:1385‐96. - PubMed
Garcia‐Borreguero 2013
-
- Garcia‐Borreguero D, Kohnen R, Silber MH, Winkelman JW, Earley CJ, Hogl B, et al. The long‐term treatment of restless legs syndrome/Willis‐Ekbom disease: evidence‐based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Medicine 2013;14:675‐84. - PubMed
Guy 1976
-
- Guy W. ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare, 1976.
Guyatt 2011
-
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64:1277‐82. - PubMed
Hays 1992
-
- Hays RD, Stewart AL. Sleep measures. Measuring functioning and well being: the Medical Outcomes Study approach. Durham, NC: Duke University Press, 1992.
Hening 2003
-
- Hening WA, Allen RP, Thanner S, Washburn T, Heckler D, Walter AS, et al. The Johns Hopkins telephone diagnostic interview for the restless legs syndrome: preliminary investigation for validation in a multi‐center patient and control population. Sleep Medicine 2003;4(2):137‐41. - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Wiley‐Blackwell.
Hornyak 2006
-
- Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Medicine Reviews 2006;10(3):169‐77. - PubMed
Johns 1991
-
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale.. Sleep 1991;14(6):540‐5. - PubMed
Kohnen 2004
-
- Kohnen R, Stiasny‐Kolster K, Oertel WH, Benes H, Trenkwalder C. Severity rating of restless legs syndrome: Validation of the RLS‐6 scale. Sleep 2004;27(Suppl 1):A342.
Krupp 1989
-
- Krupp LB, LaRocca NG, Muir‐Nash J, Steinberg AD. The fatigue severity scale. Archives of Neurology 1989;46:1121‐3. - PubMed
Montplaisir 1997
-
- Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lesperance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome. Movement Disorders 1997;12:61‐5. - PubMed
Nelson 1997
-
- Nelson C, Erikson K, Piñero DJ, Beard JL. In vivo dopamine metabolism is altered in iron‐deficient anemic rats. Journal of Nutrition 1997;127:2282‐8. - PubMed
Rye 2015
-
- Rye DB. The molecular genetics of restless legs syndrome. Sleep Medicine Clinics 2015;10(3):227‐33. - PubMed
Scholz 2011
Schormair 2008
-
- Schormair B, Kemlink D, Roeske D, Eckstein G, Xiong L, Lichtner P, et al. PTPRD (protein tyrosine phosphatase receptor type delta) is associated with restless legs syndrome. Nature Genetics 2008;40(8):946‐8. - PubMed
Silber 2013
-
- Silber MH, Becker PM, Earley C, Garcia‐Borreguero D, Ondo WG. Willis‐Ekbom Disease Foundation revised consensus statement on the management of restless legs syndrome. Mayo Clinic Proceedings 2013;88(9):977‐86. - PubMed
Stefansson 2007
-
- Stefansson H, Rye DB, Hicks A, Petursson H, Ingason A, Thorgeirsson TE, et al. A genetic risk factor for periodic limb movements in sleep. New England Journal of Medicine 2007;357(7):639‐47. - PubMed
Trenkwalder 2008
-
- Trenkwalder C, Hogl B, Benes H, Kohnen R. Augmentation in restless legs syndrome is associated with low ferritin. Sleep Medicine 2008;9(5):572‐4. - PubMed
Trotti 2008
-
- Trotti LM, Bhadriraju S, Rye DB. An update on the pathophysiology and genetics of restless legs syndrome. Current Neurology and Neuroscience Reports 2008;8:281‐7. - PubMed
Trotti 2009
-
- Trotti LM, Bliwise DL, Greer SA, Sigurdsson AP, Gudmundsdottir GB, Wessel T, et al. Correlates of PLMS variability over multiple nights and impact upon RLS diagnosis. Sleep Medicine 2009;10(6):668‐71. - PubMed
Walters 1995
-
- Walters AS. Toward a better definition of the restless legs syndrome. The International Restless Legs Syndrome Study Group. Movement Disorders 1995;10(5):634‐42. - PubMed
Walters 2003
-
- Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Medicine 2003;4(2):121‐32. - PubMed
Wan 2014
Ware 1992
-
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care 1992;30(6):473‐83. - PubMed
Wilt 2013
-
- Wilt TJ, MacDonald R, Ouellette J, Khawaja IS, Rutks I, Butler M, et al. Pharmacologic therapy for primary restless legs syndrome: a systematic review and meta‐analysis. JAMA Internal Medicine 2013;173(7):496‐505. - PubMed
Winkelman 2016
-
- Winkelman JW, Armstrong MJ, Allen RP, Chaudhuri KR, Ondo W, Trenkwalder C, et al. Practice guideline summary: Treatment of restless legs syndrome in adults: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2016;87(24):2585‐93. - PMC - PubMed
Winkelmann 2007
-
- Winkelmann J, Schormair B, Lichtner P, Ripke S, Xiong L, Jalilzadeh S, et al. Genome‐wide association study of restless legs syndrome identifies common variants in three genomic regions. Nature Genetics 2007;39(8):1000‐6. - PubMed
References to other published versions of this review
Trotti 2009b
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

