Inhaled Nanoformulated mRNA Polyplexes for Protein Production in Lung Epithelium
- PMID: 30609147
- PMCID: PMC7490222
- DOI: 10.1002/adma.201805116
Inhaled Nanoformulated mRNA Polyplexes for Protein Production in Lung Epithelium
Abstract
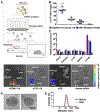
Noninvasive aerosol inhalation is an established method of drug delivery to the lung, and remains a desirable route for nucleic-acid-based therapeutics. In vitro transcribed (IVT) mRNA has broad therapeutic applicability as it permits temporal and dose-dependent control of encoded protein expression. Inhaled delivery of IVT-mRNA has not yet been demonstrated and requires development of safe and effective materials. To meet this need, hyperbranched poly(beta amino esters) (hPBAEs) are synthesized to enable nanoformulation of stable and concentrated polyplexes suitable for inhalation. This strategy achieves uniform distribution of luciferase mRNA throughout all five lobes of the lung and produces 101.2 ng g-1 of luciferase protein 24 h after inhalation of hPBAE polyplexes. Importantly, delivery is localized to the lung, and no luminescence is observed in other tissues. Furthermore, using an Ai14 reporter mouse model it is identified that 24.6% of the total lung epithelial cell population is transfected after a single dose. Repeat dosing of inhaled hPBAE-mRNA generates consistent protein production in the lung, without local or systemic toxicity. The results indicate that nebulized delivery of IVT-mRNA facilitated by hPBAE vectors may provide a clinically relevant delivery system to lung epithelium.
Keywords: biomaterials; gene delivery; inhalation; messenger RNA; topology.
© 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflict of Interest
A patent for the materials developed in this manuscript has been filed by A.K.P., J.C.K., K.J.K., and D.G.A.
Figures



References
-
- Sahin U, Karikó K, Türeci Ö, Nat. Rev. Drug Discovery 2014, 13, 759. - PubMed
-
- Kormann MSD, Hasenpusch G, Aneja MK, Nica G, Flemmer AW, Herber-Jonat S, Huppmann M, Mays LE, Illenyi M, Schams A, Griese M, Bittmann I, Handgretinger R, Hartl D, Rosenecker J, Rudolph C, Nat. Biotechnol 2011, 29, 154. - PubMed
-
- Beck SE, Laube BL, Barberena CI, Fischer AC, Adams RJ, Chesnut K, Flotte TR, Guggino WB, Mol. Ther 2002, 6, 546. - PubMed
-
- Alton EWFW, Armstrong DK, Ashby D, Bayfield KJ, Bilton D, Bloomfield EV, Boyd AC, Brand J, Buchan R, Calcedo R, Carvelli P, Chan M, Cheng SH, Collie DDS, Cunningham S, Davidson HE, Davies G, Davies JC, Davies LA, Dewar MH, Doherty A, Donovan J, Dwyer NS, Elgmati HI, Featherstone RF, Gavino J, Gea-Sorli S, Geddes DM, Gibson JSR, Gill DR, Greening AP, Griesenbach U, Hansell DM, Harman K, Higgins TE, Hodges SL, Hyde SC, Hyndman L, Innes JA, Jacob J, Jones N, Keogh BF, Limberis MP, Lloyd-Evans P, Maclean AW, Manvell MC, McCormick D, McGovern M, McLachlan G, Meng C, Montero MA, Milligan H, Moyce LJ, Murray GD, Nicholson AG, Osadolor T, Parra-Leiton J, Porteous DJ, Pringle IA, Punch EK, Pytel KM, Quittner AL, Rivellini G, Saunders CJ, Scheule RK, Sheard S, Simmonds NJ, Smith K, Smith SN, Soussi N, Soussi S, Spearing EJ, Stevenson BJ, Sumner-Jones SG, Turkkila M, Ureta RP, Waller MD, Wasowicz MY, Wilson JM, Wolstenholme-Hogg P, Lancet Respir. Med 2015, 3, 684. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

