The impact of GPIbα on platelet-targeted FVIII gene therapy in hemophilia A mice with pre-existing anti-FVIII immunity
- PMID: 30609275
- PMCID: PMC6397061
- DOI: 10.1111/jth.14379
The impact of GPIbα on platelet-targeted FVIII gene therapy in hemophilia A mice with pre-existing anti-FVIII immunity
Abstract
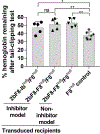
Essentials Platelet-specific FVIII gene therapy is effective in hemophilia A mice even with inhibitors. The impact of platelet adherence via VWF/GPIbα binding on platelet gene therapy was investigated. GPIbα does not significantly affect platelet gene therapy of hemophilia A with inhibitors. Platelet gene therapy induces immune tolerance in hemophilia A mice with pre-existing immunity. SUMMARY: Background We have previously demonstrated that von Willebrand factor (VWF) is essential in platelet-specific FVIII (2bF8) gene therapy of hemophilia A (HA) with inhibitory antibodies (inhibitors). At the site of injury, platelet adherence is initiated by VWF binding to the platelet GPIb complex. Objective To investigate the impact of GPIbα on platelet gene therapy of HA with inhibitors. Methods Platelet-FVIII expression was introduced by 2bF8 lentivirus (2bF8LV) transduction of hematopoietic stem cells (HSCs) from GPIbαnull (Ibnull ) mice or rhF8-primed FVIIInull (F8null ) mice followed by transplantation into lethally irradiated rhF8-primed F8null recipients. Animals were analyzed by flow cytometry, FVIII assays and the tail bleeding test. Results After transplantation, 99% of platelets were derived from donors. The macrothrombocytopenia phenotype was maintained in F8null mice that received 2bF8LV-transduced Ibnull HSCs (2bF8-Ibnull /F8null ). The platelet-FVIII expression level in 2bF8-Ibnull /F8null recipients was similar to that obtained from F8null mice that received 2bF8LV-transduced F8null HSCs (2bF8-F8null /F8null ). The tail bleeding test showed that the remaining hemoglobin level in the 2bF8-Ibnull /F8null group was significantly higher than in the F8null control group, but there was no significant difference between the 2bF8-Ibnull /F8null and 2bF8-F8null /F8null groups. The half-life of inhibitor disappearance time was comparable between the 2bF8-Ibnull /F8null and 2bF8-F8null /F8null groups. The rhF8 re-challenge did not elicit a memory immune response once inhibitor titers dropped to undetectable levels after 2bF8 gene therapy. Conclusion GPIbα does not significantly impact platelet gene therapy of HA with inhibitors. 2bF8 gene therapy restores hemostasis and promotes immune tolerance in HA mice with pre-existing immunity.
Keywords: FVIII; GPIbα; gene therapy; hemophilia A; platelet.
© 2019 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
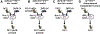
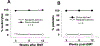
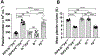


Similar articles
-
Evaluating clinically translatable conditioning for platelet gene therapy in murine hemophilia A with inhibitors.J Thromb Haemost. 2024 Nov;22(11):3035-3047. doi: 10.1016/j.jtha.2024.07.023. Epub 2024 Aug 8. J Thromb Haemost. 2024. PMID: 39127324
-
The important role of von Willebrand factor in platelet-derived FVIII gene therapy for murine hemophilia A in the presence of inhibitory antibodies.J Thromb Haemost. 2015 Jul;13(7):1301-9. doi: 10.1111/jth.13001. Epub 2015 Jun 11. J Thromb Haemost. 2015. PMID: 25955153 Free PMC article.
-
Immune tolerance induced by platelet-targeted factor VIII gene therapy in hemophilia A mice is CD4 T cell mediated.J Thromb Haemost. 2017 Oct;15(10):1994-2004. doi: 10.1111/jth.13800. Epub 2017 Sep 11. J Thromb Haemost. 2017. PMID: 28799202 Free PMC article.
-
Platelet-Targeted FVIII Gene Therapy Restores Hemostasis and Induces Immune Tolerance for Hemophilia A.Front Immunol. 2020 Jun 12;11:964. doi: 10.3389/fimmu.2020.00964. eCollection 2020. Front Immunol. 2020. PMID: 32595633 Free PMC article. Review.
-
Platelet and endothelial expression of clotting factors for the treatment of hemophilia.Thromb Res. 2012 May;129 Suppl 2(Suppl 2):S46-8. doi: 10.1016/j.thromres.2012.02.031. Epub 2012 Mar 14. Thromb Res. 2012. PMID: 22421106 Free PMC article. Review.
Cited by
-
Platelets in Alcohol-Associated Liver Disease: Interaction With Neutrophils.Cell Mol Gastroenterol Hepatol. 2024;18(1):41-52. doi: 10.1016/j.jcmgh.2024.03.001. Epub 2024 Mar 8. Cell Mol Gastroenterol Hepatol. 2024. PMID: 38461963 Free PMC article. Review.
-
Unexpected enhancement of FVIII immunogenicity by endothelial expression in lentivirus-transduced and transgenic mice.Blood Adv. 2020 May 26;4(10):2272-2285. doi: 10.1182/bloodadvances.2020001468. Blood Adv. 2020. PMID: 32453842 Free PMC article.
-
Targeting transmembrane-domain-less MOG expression to platelets prevents disease development in experimental autoimmune encephalomyelitis.Front Immunol. 2022 Oct 27;13:1029356. doi: 10.3389/fimmu.2022.1029356. eCollection 2022. Front Immunol. 2022. PMID: 36389708 Free PMC article.
-
Escape or Fight: Inhibitors in Hemophilia A.Front Immunol. 2020 Mar 24;11:476. doi: 10.3389/fimmu.2020.00476. eCollection 2020. Front Immunol. 2020. PMID: 32265927 Free PMC article. Review.
-
Evaluating clinically translatable conditioning for platelet gene therapy in murine hemophilia A with inhibitors.J Thromb Haemost. 2024 Nov;22(11):3035-3047. doi: 10.1016/j.jtha.2024.07.023. Epub 2024 Aug 8. J Thromb Haemost. 2024. PMID: 39127324
References
-
- George JN. Platelets. Lancet 2000; 355: 1531–9. - PubMed
-
- Nash GF, Turner LF, Scully MF, Kakkar AK. Platelets and cancer. Lancet Oncol 2002; 3: 425–30. - PubMed
-
- Xu XR, Yousef GM, Ni H. Cancer and platelet crosstalk: opportunities and challenges for aspirin and other antiplatelet agents. Blood 2018; 131: 1777–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

