Mutations in NCAPG2 Cause a Severe Neurodevelopmental Syndrome that Expands the Phenotypic Spectrum of Condensinopathies
- PMID: 30609410
- PMCID: PMC6323578
- DOI: 10.1016/j.ajhg.2018.11.017
Mutations in NCAPG2 Cause a Severe Neurodevelopmental Syndrome that Expands the Phenotypic Spectrum of Condensinopathies
Abstract
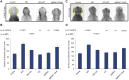
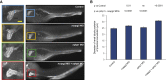
The use of whole-exome and whole-genome sequencing has been a catalyst for a genotype-first approach to diagnostics. Under this paradigm, we have implemented systematic sequencing of neonates and young children with a suspected genetic disorder. Here, we report on two families with recessive mutations in NCAPG2 and overlapping clinical phenotypes that include severe neurodevelopmental defects, failure to thrive, ocular abnormalities, and defects in urogenital and limb morphogenesis. NCAPG2 encodes a member of the condensin II complex, necessary for the condensation of chromosomes prior to cell division. Consistent with a causal role for NCAPG2, we found abnormal chromosome condensation, augmented anaphase chromatin-bridge formation, and micronuclei in daughter cells of proband skin fibroblasts. To test the functional relevance of the discovered variants, we generated an ncapg2 zebrafish model. Morphants displayed clinically relevant phenotypes, such as renal anomalies, microcephaly, and concomitant increases in apoptosis and altered mitotic progression. These could be rescued by wild-type but not mutant human NCAPG2 mRNA and were recapitulated in CRISPR-Cas9 F0 mutants. Finally, we noted that the individual with a complex urogenital defect also harbored a heterozygous NPHP1 deletion, a common contributor to nephronophthisis. To test whether sensitization at the NPHP1 locus might contribute to a more severe renal phenotype, we co-suppressed nphp1 and ncapg2, which resulted in significantly more dysplastic renal tubules in zebrafish larvae. Together, our data suggest that impaired function of NCAPG2 results in a severe condensinopathy, and they highlight the potential utility of examining candidate pathogenic lesions beyond the primary disease locus.
Keywords: NCAPD3; NCAPH2; NPHP1; cell cycle delay; chromosome condensation; genetic interaction; microcephaly; micronuclei; renal cyst; zebrafish.
Copyright © 2018 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Combined deletion of two Condensin II system genes (NCAPG2 and MCPH1) in a case of severe microcephaly and mental deficiency.Eur J Med Genet. 2013 Nov;56(11):635-41. doi: 10.1016/j.ejmg.2013.07.007. Epub 2013 Sep 4. Eur J Med Genet. 2013. PMID: 24013099
-
Mutations in TMEM260 Cause a Pediatric Neurodevelopmental, Cardiac, and Renal Syndrome.Am J Hum Genet. 2017 Apr 6;100(4):666-675. doi: 10.1016/j.ajhg.2017.02.007. Epub 2017 Mar 16. Am J Hum Genet. 2017. PMID: 28318500 Free PMC article.
-
Bi-allelic Variants in DYNC1I2 Cause Syndromic Microcephaly with Intellectual Disability, Cerebral Malformations, and Dysmorphic Facial Features.Am J Hum Genet. 2019 Jun 6;104(6):1073-1087. doi: 10.1016/j.ajhg.2019.04.002. Epub 2019 May 9. Am J Hum Genet. 2019. PMID: 31079899 Free PMC article.
-
Taking cohesin and condensin in context.PLoS Genet. 2018 Jan 25;14(1):e1007118. doi: 10.1371/journal.pgen.1007118. eCollection 2018 Jan. PLoS Genet. 2018. PMID: 29370184 Free PMC article. Review.
-
Three-step model for condensin activation during mitotic chromosome condensation.Cell Cycle. 2010 Aug 15;9(16):3243-55. doi: 10.4161/cc.9.16.12620. Epub 2010 Aug 7. Cell Cycle. 2010. PMID: 20703077 Review.
Cited by
-
Causal and Candidate Gene Variants in a Large Cohort of Women With Primary Ovarian Insufficiency.J Clin Endocrinol Metab. 2022 Feb 17;107(3):685-714. doi: 10.1210/clinem/dgab775. J Clin Endocrinol Metab. 2022. PMID: 34718612 Free PMC article.
-
Biallelic loss-of-function variants in CACHD1 cause a novel neurodevelopmental syndrome with facial dysmorphism and multisystem congenital abnormalities.Genet Med. 2024 Apr;26(4):101057. doi: 10.1016/j.gim.2023.101057. Epub 2023 Dec 27. Genet Med. 2024. PMID: 38158856 Free PMC article.
-
Congenital anterior segment ocular disorders: Genotype-phenotype correlations and emerging novel mechanisms.Prog Retin Eye Res. 2024 Sep;102:101288. doi: 10.1016/j.preteyeres.2024.101288. Epub 2024 Aug 2. Prog Retin Eye Res. 2024. PMID: 39097141 Free PMC article. Review.
-
Single-cell transcription profiles in Bloom syndrome patients link BLM deficiency with altered condensin complex expression signatures.Hum Mol Genet. 2022 Jul 7;31(13):2185-2193. doi: 10.1093/hmg/ddab373. Hum Mol Genet. 2022. PMID: 35099000 Free PMC article.
-
miR-638 suppresses cervical cancer progression by inhibiting NCAPG2 under the treatment of Tetrandrine.Histol Histopathol. 2024 Apr;39(4):497-509. doi: 10.14670/HH-18-657. Epub 2023 Aug 4. Histol Histopathol. 2024. PMID: 37702425
References
-
- Thapar A., Cooper M., Rutter M. Neurodevelopmental disorders. Lancet Psychiatry. 2017;4:339–346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

