Relevance of Autophagy in Parenchymal and Non-Parenchymal Liver Cells for Health and Disease
- PMID: 30609663
- PMCID: PMC6357193
- DOI: 10.3390/cells8010016
Relevance of Autophagy in Parenchymal and Non-Parenchymal Liver Cells for Health and Disease
Abstract
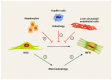
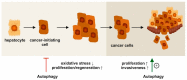
Autophagy is a highly conserved intracellular process for the ordered degradation and recycling of cellular components in lysosomes. In the liver, parenchymal cells (i.e., mainly hepatocytes) utilize autophagy to provide amino acids, glucose, and free fatty acids as sources of energy and biosynthesis functions, but also for recycling and controlling organelles such as mitochondria. Non-parenchymal cells of the liver, including endothelial cells, macrophages (Kupffer cells), and hepatic stellate cells (HSC), also employ autophagy, either for maintaining cellular homeostasis (macrophages, endothelium) or for providing energy for their activation (stellate cells). In hepatocytes, autophagy contributes to essential homeostatic functions (e.g., gluconeogenesis, glycogenolysis, fatty acid oxidation), but is also implicated in diseases. For instance, storage disorders (alpha 1 antitrypsin deficiency, Wilson's disease), metabolic (non-alcoholic steatohepatitis, NASH), and toxic (alcohol) liver diseases may benefit from augmenting autophagy in hepatocytes. In hepatic fibrosis, autophagy has been implicated in the fibrogenic activation of HSC to collagen-producing myofibroblasts. In hepatocellular carcinoma (HCC), autophagy may contribute to tumor surveillance as well as invasiveness, indicating a dual and stage-dependent function in cancer. As many drugs directly or indirectly modulate autophagy, it is intriguing to investigate autophagy-targeting, possibly even cell type-directed strategies for the treatment of hereditary liver diseases, NASH, fibrosis, and HCC.
Keywords: biomarkers; cirrhosis; fibrosis; hepatic stellate cells; hepatocellular carcinoma; hepatocytes; macrophages; sinusoidal endothelial cells.
Conflict of interest statement
The authors declare no conflict of interest related to this article. However, work in the laboratory of F.T. has been supported by funding from Allergan, Galapagos, Inventiva, and Bristol Myers Squibb. The laboratory of R.W. cooperates with Silence Therapeutics. The funders or cooperation companies had no role in the design of the study, in the writing of the manuscript, or in the decision to publish this review.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

