mTOR: A Cellular Regulator Interface in Health and Disease
- PMID: 30609721
- PMCID: PMC6356367
- DOI: 10.3390/cells8010018
mTOR: A Cellular Regulator Interface in Health and Disease
Abstract
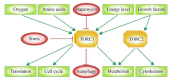
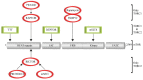
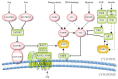
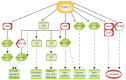
The mechanistic target of Rapamycin (mTOR) is a ubiquitously-conserved serine/threonine kinase, which has a central function in integrating growth signals and orchestrating their physiologic effects on cellular level. mTOR is the core component of differently composed signaling complexes that differ in protein composition and molecular targets. Newly identified classes of mTOR inhibitors are being developed to block autoimmune diseases and transplant rejections but also to treat obesity, diabetes, and different types of cancer. Therefore, the selective and context-dependent inhibition of mTOR activity itself might come into the focus as molecular target to prevent severe diseases and possibly to extend life span. This review provides a general introduction to the molecular composition and physiologic function of mTOR complexes as part of the Special Issue "2018 Select Papers by Cells' Editorial Board Members".
Keywords: aging; autophagy; cancer; kinase; mTOR; phosphorylation.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures
Similar articles
-
mTOR activation is a biomarker and a central pathway to autoimmune disorders, cancer, obesity, and aging.Ann N Y Acad Sci. 2015 Jun;1346(1):33-44. doi: 10.1111/nyas.12756. Epub 2015 Apr 23. Ann N Y Acad Sci. 2015. PMID: 25907074 Free PMC article. Review.
-
mTOR in Lung Neoplasms.Pathol Oncol Res. 2020 Jan;26(1):35-48. doi: 10.1007/s12253-020-00796-1. Epub 2020 Feb 3. Pathol Oncol Res. 2020. PMID: 32016810 Review.
-
The mTOR Kinase Inhibitor CZ415 Inhibits Human Papillary Thyroid Carcinoma Cell Growth.Cell Physiol Biochem. 2018;46(2):579-590. doi: 10.1159/000488625. Epub 2018 Mar 28. Cell Physiol Biochem. 2018. PMID: 29617677
-
Unmasking the impact of Rictor in cancer: novel insights of mTORC2 complex.Carcinogenesis. 2018 Jul 30;39(8):971-980. doi: 10.1093/carcin/bgy086. Carcinogenesis. 2018. PMID: 29955840 Review.
-
mTOR: Role in cancer, metastasis and drug resistance.Semin Cancer Biol. 2019 Dec;59:92-111. doi: 10.1016/j.semcancer.2019.07.003. Epub 2019 Aug 10. Semin Cancer Biol. 2019. PMID: 31408724 Review.
Cited by
-
Cellular and Molecular Mechanisms Involved in Hematopoietic Stem Cell Aging as a Clinical Prospect.Oxid Med Cell Longev. 2022 Apr 1;2022:2713483. doi: 10.1155/2022/2713483. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35401928 Free PMC article. Review.
-
The Key Roles of PTEN in T-Cell Acute Lymphoblastic Leukemia Development, Progression, and Therapeutic Response.Cancers (Basel). 2019 May 6;11(5):629. doi: 10.3390/cancers11050629. Cancers (Basel). 2019. PMID: 31064074 Free PMC article. Review.
-
Manipulation of Cellular Processes via Nucleolus Hijaking in the Course of Viral Infection in Mammals.Cells. 2021 Jun 25;10(7):1597. doi: 10.3390/cells10071597. Cells. 2021. PMID: 34202380 Free PMC article. Review.
-
Glucose metabolism and function of CD4+ Tregs are regulated by the TLR8/mTOR signal in an environment of SKOV3 cell growth.Cancer Med. 2023 Aug;12(15):16310-16322. doi: 10.1002/cam4.6247. Epub 2023 Jun 14. Cancer Med. 2023. PMID: 37317670 Free PMC article.
-
Lifetime Impact of Cow's Milk on Overactivation of mTORC1: From Fetal to Childhood Overgrowth, Acne, Diabetes, Cancers, and Neurodegeneration.Biomolecules. 2021 Mar 9;11(3):404. doi: 10.3390/biom11030404. Biomolecules. 2021. PMID: 33803410 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

