Living in Promiscuity: The Multiple Partners of Alpha-Synuclein at the Synapse in Physiology and Pathology
- PMID: 30609739
- PMCID: PMC6337145
- DOI: 10.3390/ijms20010141
Living in Promiscuity: The Multiple Partners of Alpha-Synuclein at the Synapse in Physiology and Pathology
Abstract
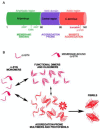
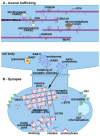
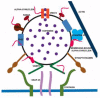
Alpha-synuclein (α-syn) is a small protein that, in neurons, localizes predominantly to presynaptic terminals. Due to elevated conformational plasticity, which can be affected by environmental factors, in addition to undergoing disorder-to-order transition upon interaction with different interactants, α-syn is counted among the intrinsically disordered proteins (IDPs) family. As with many other IDPs, α-syn is considered a hub protein. This function is particularly relevant at synaptic sites, where α-syn is abundant and interacts with many partners, such as monoamine transporters, cytoskeletal components, lipid membranes, chaperones and synaptic vesicles (SV)-associated proteins. These protein⁻protein and protein⁻lipid membrane interactions are crucial for synaptic functional homeostasis, and alterations in α-syn can cause disruption of this complex network, and thus a failure of the synaptic machinery. Alterations of the synaptic environment or post-translational modification of α-syn can induce its misfolding, resulting in the formation of oligomers or fibrillary aggregates. These α-syn species are thought to play a pathological role in neurodegenerative disorders with α-syn deposits such as Parkinson's disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA), which are referred to as synucleinopathies. Here, we aim at revising the complex and promiscuous role of α-syn at synaptic terminals in order to decipher whether α-syn molecular interactants may influence its conformational state, contributing to its aggregation, or whether they are just affected by it.
Keywords: conformational plasticity; interactome; synaptic proteins; synucleinopathies; α-synuclein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kahle P.J., Neumann M., Ozmen L., Muller V., Jacobsen H., Schindzielorz A., Okochi M., Leimer U., van Der Putten H., Probst A., et al. Subcellular localization of wild-type and Parkinson’s disease-associated mutant alpha -synuclein in human and transgenic mouse brain. J. Neurosci. 2000;20:6365–6373. doi: 10.1523/JNEUROSCI.20-17-06365.2000. - DOI - PMC - PubMed
-
- Abeliovich A., Schmitz Y., Farinas I., Choi-Lundberg D., Ho W.H., Castillo P.E., Shinsky N., Verdugo J.M., Armanini M., Ryan A., et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/S0896-6273(00)80886-7. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

