(-)-Epigallocatechin-3-Gallate Decreases Osteoclastogenesis via Modulation of RANKL and Osteoprotegrin
- PMID: 30609798
- PMCID: PMC6337469
- DOI: 10.3390/molecules24010156
(-)-Epigallocatechin-3-Gallate Decreases Osteoclastogenesis via Modulation of RANKL and Osteoprotegrin
Abstract
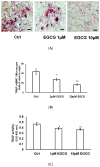
Osteoporosis is the second most common epidemiologic disease in the aging population worldwide. Previous studies have found that frequent tea drinkers have higher bone mineral density and less hip fracture. We previously found that (-)-epigallocatechin gallate (EGCG) (20⁻100 µmol/L) significantly suppressed receptor activator of nuclear factor-kB ligand (RANKL)-induced osteoclastogenesis and pit formation via inhibiting NF-κB transcriptional activity and nuclear transport of NF-κB in RAW 264.7 cells and murine primary bone marrow macrophage cells. The most important regulation in osteoclastogenesis is the receptor activator of nuclear factor-kB/RANKL/osteoprotegrin (RANK/RANKL/OPG) pathway. In this study, we used the coculture of RAW 264.7 cells and the feeder cells, ST2, to evaluate how EGCG regulated the RANK/RANKL/OPG pathway in RAW 264.7 cells and ST2 cells. We found EGCG decreased the RANKL/OPG ratio in both mRNA expression and secretory protein levels and eventually decreased osteoclastogenesis by TRAP (+) stain osteoclasts and TRAP activity at low concentrations-1 and 10 µmol/L-via the RANK/RANKL/OPG pathway. The effective concentration can be easily achieved in daily tea consumption. Taken together, our results implicate that EGCG could be an important nutrient in modulating bone resorption.
Keywords: EGCG; RANK/RANKL/OPG; RAW 264.7 cells; catechin; osteoclast; osteoclastogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Liao S., Kao Y.H., Hiipakka R.A. Green tea: Biochemical and biological basis for health benefits. Vitam. Horm. 2001;62:1–94. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

