Magnesium Implants: Prospects and Challenges
- PMID: 30609830
- PMCID: PMC6337251
- DOI: 10.3390/ma12010136
Magnesium Implants: Prospects and Challenges
Abstract
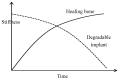
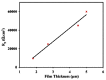
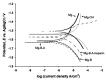
Owing to their suitable mechanical property and biocompatibility as well as the technological possibility of controlling their high corrosion rates, magnesium and its alloys have attracted significant attention as temporary bio-implants. Though the ability of magnesium to harmlessly biodegrade and its inherent biocompatibility make magnesium alloys a suitable choice for a temporary implant, their high corrosion rates limit their practical application, as the implants can potentially corrode away even before the healing process has completed. Different approaches, such as alloying, surface modification, and conversion coatings, have been explored to improve the corrosion resistance of various magnesium alloys. However, the corrosion behavior of magnesium implants with and without a surface modification has been generally investigated under in-vitro conditions, and studies under in-vivo conditions are limited, which has contributed to the lack of translation of magnesium implants in practical applications. This paper comprehensively reviews the prospects of magnesium alloy implants and the current challenges due to their rapid degradation in a physiological environment. This paper also provides a comprehensive review of the corrosion mitigation measures for these temporary implants.
Keywords: corrosion; implant; magnesium alloy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










References
-
- Mushahary D. Surface Functionalisation of Magnesium Alloys for Use as Bio-Implants. Institute for Frontier Materials, Deakin University; Geelong, Australia: 2014.
-
- Niinomi M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A. 2002;33:477. doi: 10.1007/s11661-002-0109-2. - DOI
-
- Williams D.F. Corrosion of Implant Materials. Annu. Rev. Mater. Sci. 1976;6:237–266. doi: 10.1146/annurev.ms.06.080176.001321. - DOI
-
- Nielsen K. Corrosion of metallic implants. Br. Corros. J. 1987;22:272–278. doi: 10.1179/000705987798271352. - DOI
Publication types
LinkOut - more resources
Full Text Sources

