Nanoheterostructures (NHS) and Their Applications in Nanomedicine: Focusing on In Vivo Studies
- PMID: 30609839
- PMCID: PMC6337150
- DOI: 10.3390/ma12010139
Nanoheterostructures (NHS) and Their Applications in Nanomedicine: Focusing on In Vivo Studies
Abstract
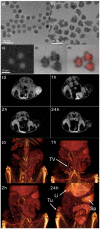
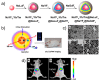
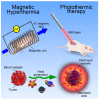
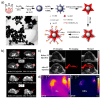
Inorganic nanoparticles have great potential for application in many fields, including nanomedicine. Within this class of materials, inorganic nanoheterostructures (NHS) look particularly promising as they can be formulated as the combination of different domains; this can lead to nanosystems with different functional properties, which, therefore, can perform different functions at the same time. This review reports on the latest development in the synthesis of advanced NHS for biomedicine and on the tests of their functional properties in in vivo studies. The literature discussed here focuses on the diagnostic and therapeutic applications with special emphasis on cancer. Considering the diagnostics, a description of the NHS for cancer imaging and multimodal imaging is reported; more specifically, NHS for magnetic resonance, computed tomography and luminescence imaging are considered. As for the therapeutics, NHS employed in magnetic hyperthermia or photothermal therapies are reported. Examples of NHS for cancer theranostics are also presented, emphasizing their dual usability in vivo, as imaging and therapeutic tools. Overall, NHS show a great potential for biomedicine application; further studies, however, are necessary regarding the safety associated to their use.
Keywords: biomedicine; hyperthermia; imaging; in vivo testing; nanoheterostructures; photothermal therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Engineering Persistent Luminescence Nanoparticles for Biological Applications: From Biosensing/Bioimaging to Theranostics.Acc Chem Res. 2018 May 15;51(5):1131-1143. doi: 10.1021/acs.accounts.7b00619. Epub 2018 Apr 17. Acc Chem Res. 2018. PMID: 29664602
-
Design and Functionalization of the NIR-Responsive Photothermal Semiconductor Nanomaterials for Cancer Theranostics.Acc Chem Res. 2017 Oct 17;50(10):2529-2538. doi: 10.1021/acs.accounts.7b00294. Epub 2017 Oct 3. Acc Chem Res. 2017. PMID: 28972736
-
Nano-Theranostics for the Sensing, Imaging and Therapy of Prostate Cancers.Front Chem. 2022 Apr 12;10:830133. doi: 10.3389/fchem.2022.830133. eCollection 2022. Front Chem. 2022. PMID: 35494646 Free PMC article. Review.
-
Multifunctional Bi@PPy-PEG Core-Shell Nanohybrids for Dual-Modal Imaging and Photothermal Therapy.ACS Appl Mater Interfaces. 2018 Jan 17;10(2):1605-1615. doi: 10.1021/acsami.7b17838. Epub 2018 Jan 3. ACS Appl Mater Interfaces. 2018. PMID: 29272573
-
Biomedical Applications of Advanced Multifunctional Magnetic Nanoparticles.J Nanosci Nanotechnol. 2015 Dec;15(12):10091-107. doi: 10.1166/jnn.2015.11691. J Nanosci Nanotechnol. 2015. PMID: 26682455 Review.
Cited by
-
Controlled release of carnosine from poly(lactic-co-glycolic acid) beads using nanomechanical magnetic trigger towards the treatment of glioblastoma.Nanoscale Adv. 2022 Apr 27;4(10):2242-2249. doi: 10.1039/d2na00032f. eCollection 2022 May 17. Nanoscale Adv. 2022. PMID: 36133698 Free PMC article.
-
Quantitative Comparison of the Light-to-Heat Conversion Efficiency in Nanomaterials Suitable for Photothermal Therapy.ACS Appl Mater Interfaces. 2022 Jul 27;14(29):33555-33566. doi: 10.1021/acsami.2c08013. Epub 2022 Jul 18. ACS Appl Mater Interfaces. 2022. PMID: 35848997 Free PMC article.
-
Non-Heating Alternating Magnetic Field Nanomechanical Stimulation of Biomolecule Structures via Magnetic Nanoparticles as the Basis for Future Low-Toxic Biomedical Applications.Nanomaterials (Basel). 2021 Aug 31;11(9):2255. doi: 10.3390/nano11092255. Nanomaterials (Basel). 2021. PMID: 34578570 Free PMC article. Review.
-
Review of the Application of Dual Drug Delivery Nanotheranostic Agents in the Diagnosis and Treatment of Liver Cancer.Molecules. 2023 Oct 10;28(20):7004. doi: 10.3390/molecules28207004. Molecules. 2023. PMID: 37894483 Free PMC article. Review.
-
ZnO nanocrystals shuttled by extracellular vesicles as effective Trojan nano-horses against cancer cells.Nanomedicine (Lond). 2019 Nov;14(21):2815-2833. doi: 10.2217/nnm-2019-0231. Epub 2019 Nov 21. Nanomedicine (Lond). 2019. PMID: 31747855 Free PMC article.
References
-
- Neoh K.G., Kang E.T. Functionalization of Inorganic Nanoparticles with Polymers for Stealth Biomedical Applications. Polym. Chem. 2011;2:747–759. doi: 10.1039/C0PY00266F. - DOI
-
- Di Corato R., Quarta A., Piacenza P., Ragusa A., Figuerola A., Buonsanti R., Cingolani R., Manna L., Pellegrino T. Water Solubilization of Hydrophobic Nanocrystals by Means of Poly(Maleic Anhydride-Alt-1-Octadecene) J. Mater. Chem. 2008;18:1991–1996. doi: 10.1039/b717801h. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

