Multitarget 1,4-Dioxane Compounds Combining Favorable D2-like and 5-HT1A Receptor Interactions with Potential for the Treatment of Parkinson's Disease or Schizophrenia
- PMID: 30609891
- PMCID: PMC8378419
- DOI: 10.1021/acschemneuro.8b00677
Multitarget 1,4-Dioxane Compounds Combining Favorable D2-like and 5-HT1A Receptor Interactions with Potential for the Treatment of Parkinson's Disease or Schizophrenia
Abstract
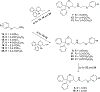
The effect of methoxy and hydroxy substitutions in different positions of the phenoxy moiety of the N-((6,6-diphenyl-1,4-dioxan-2-yl)methyl)-2-phenoxyethan-1-amine scaffold on the affinity/activity for D2-like, 5-HT1A, and α1-adrenoceptor subtypes was evaluated. Multitarget compounds with suitable combinations of dopaminergic and serotoninergic profiles were discovered. In particular, the 2-methoxy derivative 3 showed a multitarget combination of 5-HT1A/D4 agonism and D2/D3/5-HT2A antagonism, which may be a favorable profile for the treatment of schizophrenia. Interestingly, the 3-hydroxy derivative 8 behaved as a partial agonist at D2 and as a potent full agonist at D3 and D4 subtypes. In addition to its potent 5-HT1A receptor agonism, such a dopaminergic profile makes 8 a potential multitarget compound for the treatment of Parkinson's disease (PD). Indeed, the activation of 5-HT1A receptors might be helpful in reducing dyskinetic side effects associated with dopaminergic stimulation.
Keywords: 1,4-dioxane derivatives; Parkinson’s disease; Serotonin receptors; dopamine receptors; multitarget agents; schizophrenia.
Figures
Similar articles
-
Preclinical pharmacodynamic and pharmacokinetic characterization of the major metabolites of cariprazine.Drug Des Devel Ther. 2019 Sep 16;13:3229-3248. doi: 10.2147/DDDT.S188760. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31571826 Free PMC article.
-
Combined serotonin (5-HT)1A agonism, 5-HT(2A) and dopamine D₂ receptor antagonism reproduces atypical antipsychotic drug effects on phencyclidine-impaired novel object recognition in rats.Behav Brain Res. 2015 May 15;285:165-75. doi: 10.1016/j.bbr.2014.09.040. Epub 2014 Oct 16. Behav Brain Res. 2015. PMID: 25448429
-
F15063, a potential antipsychotic with dopamine D2/D3 receptor antagonist, 5-HT1A receptor agonist and dopamine D4 receptor partial agonist properties: influence on neuronal firing and neurotransmitter release.Eur J Pharmacol. 2009 Apr 1;607(1-3):74-83. doi: 10.1016/j.ejphar.2009.02.001. Eur J Pharmacol. 2009. PMID: 19326477
-
Serotonin receptors: their key role in drugs to treat schizophrenia.Prog Neuropsychopharmacol Biol Psychiatry. 2003 Oct;27(7):1159-72. doi: 10.1016/j.pnpbp.2003.09.010. Prog Neuropsychopharmacol Biol Psychiatry. 2003. PMID: 14642974 Review.
-
Dual ligands targeting dopamine D2 and serotonin 5-HT1A receptors as new antipsychotical or anti-Parkinsonian agents.Curr Med Chem. 2014;21(4):437-57. doi: 10.2174/09298673113206660300. Curr Med Chem. 2014. PMID: 24164194 Review.
Cited by
-
Highly Potent and Selective Dopamine D4 Receptor Antagonists Potentially Useful for the Treatment of Glioblastoma.J Med Chem. 2022 Sep 22;65(18):12124-12139. doi: 10.1021/acs.jmedchem.2c00840. Epub 2022 Sep 13. J Med Chem. 2022. PMID: 36098685 Free PMC article.
-
Recent advances in multitarget-directed ligands targeting G-protein-coupled receptors.Drug Discov Today. 2020 Sep;25(9):1682-1692. doi: 10.1016/j.drudis.2020.07.004. Epub 2020 Jul 9. Drug Discov Today. 2020. PMID: 32652312 Free PMC article. Review.
-
Scaffold Hybridization Strategy Leads to the Discovery of Dopamine D3 Receptor-Selective or Multitarget Bitopic Ligands Potentially Useful for Central Nervous System Disorders.ACS Chem Neurosci. 2021 Oct 6;12(19):3638-3649. doi: 10.1021/acschemneuro.1c00368. Epub 2021 Sep 16. ACS Chem Neurosci. 2021. PMID: 34529404 Free PMC article.
-
Receptor Ligands as Helping Hands to L-DOPA in the Treatment of Parkinson's Disease.Biomolecules. 2019 Apr 9;9(4):142. doi: 10.3390/biom9040142. Biomolecules. 2019. PMID: 30970612 Free PMC article. Review.
-
Novel Potent and Selective Dopamine D4 Receptor Piperidine Antagonists as Potential Alternatives for the Treatment of Glioblastoma.Pharmaceuticals (Basel). 2025 May 17;18(5):739. doi: 10.3390/ph18050739. Pharmaceuticals (Basel). 2025. PMID: 40430557 Free PMC article.
References
-
- Roth BL, Sheffler DJ, Kroeze WK (2004) Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat. Rev. Drug Discov 3 353–359. - PubMed
-
- Ye N, Neumeyer JL, Baldessarini RJ, Zhen X, Zhang A (2013) Update 1 of: Recent progress in development of dopamine receptor subtype-selective agents: Potential therapeutics for neurological and psychiatric disorders. Chem. Rev 113, PR123–168 and references therein. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical