Use of In-Game Rewards to Motivate Daily Self-Report Compliance: Randomized Controlled Trial
- PMID: 30609986
- PMCID: PMC6682282
- DOI: 10.2196/11683
Use of In-Game Rewards to Motivate Daily Self-Report Compliance: Randomized Controlled Trial
Abstract
Background: Encouraging individuals to report daily information such as unpleasant disease symptoms, daily activities and behaviors, or aspects of their physical and emotional state is difficult but necessary for many studies and clinical trials that rely on patient-reported data as primary outcomes. Use of paper diaries is the traditional method of completing daily diaries, but digital surveys are becoming the new standard because of their increased compliance; however, they still fall short of desired compliance levels.
Objective: Mobile games using in-game rewards offer the opportunity to increase compliance above the rates of digital diaries and paper diaries. We conducted a 5-week randomized control trial to compare the completion rates of a daily diary across 3 conditions: a paper-based participant-reported outcome diary (Paper PRO), an electronic-based participant-reported outcome diary (ePRO), and a novel ePRO diary with in-game rewards (Game-Motivated ePRO).
Methods: We developed a novel mobile game that is a combination of the idle and pet collection genres to reward individuals who complete a daily diary with an in-game reward. Overall, 197 individuals aged 6 to 24 years (male: 100 and female: 97) were enrolled in a 5-week study after being randomized into 1 of the 3 methods of daily diary completion. Moreover, 157 participants (male: 84 and female: 69) completed at least one diary and were subsequently included in analysis of compliance rates.
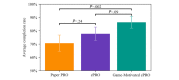
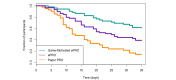
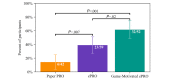
Results: We observed a significant difference (F2,124=6.341; P=.002) in compliance to filling out daily diaries, with the Game-Motivated ePRO group having the highest compliance (mean completion 86.4%, SD 19.6%), followed by the ePRO group (mean completion 77.7%, SD 24.1%), and finally, the Paper PRO group (mean completion 70.6%, SD 23.4%). The Game-Motivated ePRO (P=.002) significantly improved compliance rates above the Paper PRO. In addition, the Game-Motivated ePRO resulted in higher compliance rates than the rates of ePRO alone (P=.09). Equally important, even though we observed significant differences in completion of daily diaries between groups, we did not observe any statistically significant differences in association between the responses to a daily mood question and study group, the average diary completion time (P=.52), or the System Usability Scale score (P=.88).
Conclusions: The Game-Motivated ePRO system encouraged individuals to complete the daily diaries above the compliance rates of the Paper PRO and ePRO without altering the participants' responses.
Trial registration: ClinicalTrials.gov NCT03738254; http://clinicaltrials.gov/ct2/show/NCT03738254 (Archived by WebCite at http://www.webcitation.org/74T1p8u52).
Keywords: protocol compliance; recreational games; self-reports.
©Sara Taylor, Craig Ferguson, Fengjiao Peng, Magdalena Schoeneich, Rosalind W Picard. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 03.01.2019.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures


References
-
- Pew Research Center. 2018. Feb 05, [2018-12-10]. Mobile Fact Sheet http://www.pewinternet.org/fact-sheet/mobile/
-
- Harris C, Daniels K, Briner R. A daily diary study of goals and affective well-being at work. J Occup Organ Psychol. 2003;76:401–10. doi: 10.1348/096317903769647256. - DOI
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

