Collided ribosomes form a unique structural interface to induce Hel2-driven quality control pathways
- PMID: 30609991
- PMCID: PMC6396155
- DOI: 10.15252/embj.2018100276
Collided ribosomes form a unique structural interface to induce Hel2-driven quality control pathways
Abstract
Ribosome stalling triggers quality control pathways targeting the mRNA (NGD: no-go decay) and the nascent polypeptide (RQC: ribosome-associated quality control). RQC requires Hel2-dependent uS10 ubiquitination and the RQT complex in yeast. Here, we report that Hel2-dependent uS10 ubiquitination and Slh1/Rqt2 are crucial for RQC and NGD induction within a di-ribosome (disome) unit, which consists of the leading stalled ribosome and the following colliding ribosome. Hel2 preferentially ubiquitinated a disome over a monosome on a quality control inducing reporter mRNA in an in vitro translation reaction. Cryo-EM analysis of the disome unit revealed a distinct structural arrangement suitable for recognition and modification by Hel2. The absence of the RQT complex or uS10 ubiquitination resulted in the elimination of NGD within the disome unit. Instead, we observed Hel2-mediated cleavages upstream of the disome, governed by initial Not4-mediated monoubiquitination of eS7 and followed by Hel2-mediated K63-linked polyubiquitination. We propose that Hel2-mediated ribosome ubiquitination is required both for canonical NGD (NGDRQC+) and RQC coupled to the disome and that RQC-uncoupled NGD outside the disome (NGDRQC-) can occur in a Not4-dependent manner.
Keywords: RQT complex; no‐go mRNA decay; ribosome collision; ribosome quality control; ubiquitination.
© 2019 The Authors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Schematic drawing of reporter mRNAs containing various arrest‐inducing sequences. The box indicates GFP and HIS3 open reading frames (ORFs), and the black line indicates the untranslated region (UTR). Ribosome stalling takes place during translation of the indicated arrest sequences (shown in red) and induces an endonucleolytic cleavage to produce two fragments, the 5′ NGD intermediate (5′ NGD‐IM) and 3′ NGD intermediate (3′ NGD‐IM).
Northern blot analysis showing that Hel2 is required for endonucleolytic cleavages by R(CGN)12 arrest‐inducing sequences. The GFP‐R(CGN) 12 ‐HIS3 mRNA (FL) and the 5′ NGD‐IM were detected in the indicated strains using a DIG‐labelled GFP probe. SCR1 coding for RNA in the signal recognition particle (SRP) was used as a loading control.
Northern blot analysis showing that Ubc4 but not Ubc5 is required for endonucleolytic cleavages by R(CGN)12 arrest‐inducing sequences. The GFP‐R(CGN) 12 ‐HIS3 mRNA (FL) and the 5′ NGD‐IM were detected in the indicated strains with the DIG‐labelled GFP probe.
Northern blotting showing that Hel2 and Ubc4 are required for endonucleolytic cleavages by R(CGN)12 arrest‐inducing sequences. The GFP‐R(CGN) 12 ‐HIS3 mRNA (FL) and the 3′ NGD‐IM were detected in the indicated strains with the DIG‐labelled HIS3 probe.
Northern blotting showing that Hel2 reduces the half‐life of reporter mRNA containing R(CGN) 12 or K(AAA) 12 arrest sequences. The stability of GFP‐K(AAA) 12 ‐FLAG‐HIS3 and GFP‐R(CGN) 12 ‐FLAG‐HIS3 mRNAs was determined. The GFP‐K(AAA) 12 ‐FLAG‐HIS3 mRNA was stable in hel2 mutant cells than that in wild‐type cells (t 1/2 = 11.2 min for hel2Δ versus t 1/2 = 4.3 min for wild type). The GFP‐R(CGN) 12 ‐FLAG‐HIS3 mRNA was also stable in hel2 mutant cells than that in wild‐type cells (t 1/2 = 7.6 min for hel2 versus t 1/2 = 4.9 min for wild type). In contrast, the stability of GFP‐K(AAA) 12 ‐FLAG‐HIS3 mRNA was essentially the same in hel2 mutant cells (t 1/2 = 6.0 min) and in wild‐type cells (t 1/2 = 5.8 min). This stabilization of the reporter mRNAs that are substrate for NGD in hel2 mutant cells strongly supports the crucial role of Hel2 in NGD. On the other hand, mRNA stability did not change in slh1∆ and uS10‐K6/8R mutant cells.
Hel2 is dispensable for NSD and NMD. The GFP‐Rz mRNA is a truncated poly(A) tail‐less non‐stop mRNA that is produced by self‐cleavage of hammerhead ribozyme (Rz) and efficiently degraded in the NSD pathway (Tsuboi et al, 2012). The FLAG‐HIS3‐NS mRNA is a poly(A) tail containing nonstop mRNA and subjected to NSD (van Hoof et al, 2002). The FLAG‐his3‐100 mRNA contains premature termination codon and degraded by NMD quality control (Kuroha et al, 2009). The relative levels of reporter mRNAs in the indicated mutant cells were determined using a DIG‐labeled GFP (GFP‐Rz) or 5′ DIG‐labeled‐LNA‐FLAG (FLAG‐HIS3‐NS or FLAG‐his3‐100) probes. The relative levels of each mRNA were normalized to the mRNA level in time 0, which was assigned a value of 100, and SCR RNA levels were used as a loading control for the RNA samples.
Western blot analysis to check the expression levels of HA‐tagged Hel2 deletion mutant proteins as schematically displayed in Fig 1B using an anti‐HA antibody.
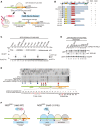
Schematic drawing of the R(CGN) 12 reporter mRNA including the two quality control pathways induced by the R(CGN)12 translation arrest sequence. Ribosome stalling occurs during translation of the R(CGN)12 arrest sequence (shown in orange) and induces RQC and NGD. In the RQC pathway, the stalled ribosome is dissociated into subunits, and peptidyl‐tRNA remaining on the 60S subunit is ubiquitinated by Ltn1 (shown in pink) and degraded by the proteasome. In the NGD pathway, an endonucleolytic cleavage produces two fragments, the 5′ NGD intermediate (5′ NGD‐IM) and 3′ NGD intermediate (3′ NGD‐IM). The green and thin grey lines indicate GFP and HIS3 open reading frames (ORFs), and the black line indicates an untranslated region (UTR).
Schematic drawing of the truncated Hel2 mutant proteins. Activities in RQC or NGD induced by the R(CGN)12 sequence are indicated.
Western blot showing that Hel2(1–315) is defective in RQC but not in NGD. The arrest products derived from the R(CGN)12 reporter in ltn1Δ cells expressing truncated Hel2 mutant protein were detected with an anti‐GFP antibody.
Northern blot showing the 5′ NGD‐IM derived from the R(CGN)12 reporter in ski2Δ cells expressing the indicated Hel2 mutant proteins. 5′ NGD‐IMs were detected with a DIG‐labelled GFP probe.
Primer extension mapping of 5′ ends of 3′ NGD intermediates in Hel2‐WT or Hel2(1–315) mutant cells at nucleotide resolution. The primer extension samples were analysed using 5% TBE‐Urea‐PAGE and detected by fluorescence. Non‐specific reverse transcription (ReTr) products are indicated by asterisks.
Dissection of NGDRQC+ and NGDRQC−: the carboxyl‐terminal region of Hel2 is required for both NGD and RQC which is likely triggered on a disome unit (pale yellow). It contains the primarily stalled, leading ribosome followed the colliding ribosome. For NGDRQC+, cleavages occur on mRNA covered by the disome, whereby the leading ribosome undergoes RQC. In the mutant Hel2 lacking the C‐terminus, an alternative NGD pathway takes place (NGDRQC−). Here, cleavages occur on mRNA covered by the ribosomes following the disome unit (blue) and the leading ribosome is not affected by RQC (grey).

- A, B
Northern blot showing that the length of 5′ NGD intermediate was altered in Hel2(1–315) expressing cells and slh1∆ cells. The full‐length GFP‐R(CGN) 12 ‐FLAG‐HIS3 mRNA and 5′ NGD intermediates (5′ NGD‐IM) or 3′ NGD intermediates (3′ NGD‐IM) were detected in the indicated mutant cells with expression of Hel2 wild‐type or 1–315 mutant from plasmid by Northern blotting with DIG‐labelled probes. 5′ NGD intermediates were detected by DIG‐labelled GFP probe in (A), and 3′ NGD intermediates were detected by the DIG‐labelled HIS3 probe in (B). SCR1 is used as loading control. FL; full length. Note an upstream shift of NGD cleavage sites in lanes A3‐4 and B3‐4.

- A
Northern blot analysis demonstrating that K63‐linked ubiquitination was required for an endonucleolytic cleavage by R(CGN)12. The ski2ΔUB‐WT and ski2Δub‐K63R cells were transformed with the R(CGN) 12 reporter, and total RNA samples were separated by 2% agarose/MOPS gel. 5′ NGD‐IMs in the cells were detected as in Fig 1D.
- B
Western blot showing that K63‐linked ubiquitination was required for RQC. Protein samples from UB‐WT, UB‐WT ltn1Δ, ub‐K63R and ub‐K63R ltn1Δ cells expressing the GFP‐R(CGN) 12 ‐HIS3 reporter were subjected to Western blot analysis using an anti‐GFP antibody to detect the arrest products. Note the accumulation of RQC‐specific CAT‐tails in lane 2.
- C
Western blot analysis demonstrating polyubiquitination of uS10: affinity‐tagged Hel2‐Flag‐TEV‐Protein A (FTP) was co‐purified with ribosomes harbouring HA‐tagged wild‐type uS10 (uS10‐3HA ribosomes) or uS10 mutated in its ubiquitination sites (K6/8R). Western blots of whole protein extracts were performed using an anti‐HA antibody to detect polyubiquitinated uS10‐3HA.
- D
Western blot analysis showing that uS10‐polyubiquitination in Hel2‐bound ribosomes was mainly K63‐linked: affinity‐tagged Hel2 (Hel2‐ProteinA‐TEV‐His6; PTH‐Hel2) was co‐purified with ribosomes from a strain expressing wild‐type ubiquitin (UB‐WT) or K63R mutant ubiquitin (ub‐K63R) and HA‐tagged uS10 (uS10‐3HA). Western blot analysis of whole protein extracts was performed using anti‐HA antibody.
- E
Western blot of in vitro polyubiquitination assays of uS10. Reactions were performed with the indicated components including His‐tagged ubiquitin and several ubiquitin mutants. Polyubiquitinated HA‐tagged uS10 (uS10‐3HA) was detected by Western blot analysis using an anti‐HA antibody.
- F–H
Northern blot analysis of NGD cleavage sites in the absence of uS10 ubiquitination or RQT complex components: the full‐length GFP‐R(CGN) 12 ‐FLAG‐HIS3 mRNA and 5′ NGD intermediates (5′ NGD‐IM) or 3′ NGD intermediates (3′ NGD‐IM) were detected in the indicated mutant cells by Northern blotting with DIG‐labelled probes. 5′ NGD intermediates were detected by DIG‐labelled GFP probe, and 3′ NGD intermediates were detected by the DIG‐labelled HIS3 probe. SCR1 was used as loading control. FL = full length. Note the upstream shift of NGD cleavage sites in lanes F4, G4, H6 and H8.
- I
Mapping of 5′ ends of 3′ NGD intermediates as demonstrated in Fig 1E. Non‐specific reverse transcription (ReTr) products are indicated by asterisks. Note that both uS10 ubiquitination and Slh1/Rqt2 were required for an endonucleolytic cleavage within the road‐blocked ribosome (X1–X4).
- J
Model for RQC‐coupled (NGDRQC+) and RQC‐uncoupled (NGDRQC−) endonucleolytic cleavages in the R(CGN)12 reporter mRNA. Endonucleolytic cleavage sites are depicted corresponding to panel (I).

- A
Top: schematic drawing of the (CGA‐CCG) dicodon reporter mRNA used for in vitro translation experiments. Bottom: scheme outlining the principle of ribosome–nascent chain (RNC) purification. Stalled and colliding ribosomes are pulled down via an affinity tag on the nascent chain.
- B, C
Both RQC and NGDRQC+ are triggered by a (CGA‐CCG) dicodon containing arrest sequence in vivo. (B) Western blot showing the arrest products derived from the GFP‐X‐FLAG‐HIS3 reporter. Translation products were detected by Western blot using an anti‐GFP antibody. (C) Northern blot for the 5′ NGD‐IM derived from the (CGA‐CCG) reporter in ski2Δ cells. 5′ NGD‐IMs were detected with a DIG‐labelled GFP probe. SCR1 was used as a load control.
- D
Western blot of test translations using the (CGA‐CCG) dicodon stalling mRNA reporter shown in (A). The mRNA reporter was added to a yeast in vitro translation extract obtained from a ski2ΔuS10‐3HA strain. Expression of the translation products (free His‐ and V5‐tagged truncated uL4 protein and the same protein attached to tRNA) was visualized with an anti‐V5 antibody.
- E
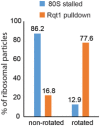
Sucrose gradient fractions (top) and Western blot analysis (bottom) of the (CGA‐CCG) dicodon‐stalled RNC samples. After the translation reaction, the RNCs were affinity‐purified as indicated in (C). The eluate was loaded on a 10–50% sucrose gradient and fractionated. Each collected fraction was analysed using anti‐HA antibody to detect uS10‐HA. Note that disomes are preferentially polyubiquitinated over monosomes.
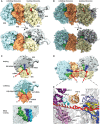
- A, B
Side view and top view of the Cryo‐EM reconstruction (A) and molecular model (B) of the disome stalled on a (CGA‐CCG) reporter mRNA. The first ribosome in the disome stalled on the dicodon mRNA is referred to as the leading ribosome and the second as the colliding ribosome.
- C
Cut top views on the leading and colliding ribosome. The leading ribosome is in a non‐rotated POST‐state containing tRNAs in P/P and E/E states and an empty A‐site, whereas the colliding ribosome is in a rotated state containing hybrid A/P and P‐/E‐site tRNAs. An overlay is shown omitting the 40S subunit for clarity.
- D
Cut top view on the disome with focus on the mRNA density highlighted in red.
- E
Zoom on the mRNA at the interface of the leading and colliding ribosome. The mRNA exits the leading ribosome close to ribosomal proteins uS11, eS26 and eS28 and enters the colliding ribosome near h16, uS3, uS5 and eS30.

mRNA was modelled into the refined maps of the hybrid A/P‐P/E tRNA containing monosome and the P/P tRNA containing monosome with ES27 in the L1 conformation (see Appendix Figs S4 and S6). These ribosomes are not involved in disome formation and show density for mRNA in covering approximately 30 nucleotides in the canonical path. In the disome, the mRNA deviates from the canonical path at the exit site of 5′ mRNA and at the entry site of the 3′‐mRNA in the colliding ribosome. In the disome, the corresponding nucleotides are stretching out to connect leading and colliding ribosome.
Superposition of the disome map shown as in Fig 4A with the isolated ES27 density from the refine monosome sub‐class representing the P/P tRNA containing ribosome with ES27 in the L1 position (blue). Note that ES27 would clash with the 60S‐40S disome bridge involving ES6b.
Left panel: superposition of the refined colliding ribosome from the disome (yellow orange) with the hybrid A/P‐P/E tRNA containing monosome (grey; see Appendix Figs S4 and S6). The view focuses on ES6. Middle panel: superposition of hybrid A/P‐P/E tRNA containing monosome with the difference map. Note that the only significant difference is in ES6c. Right panel: superposition of molecular models for ES6 in a disome (dark red/brown) and in a monosome (grey).

- A
Overview of the contact interface. The thumbnail on top indicates the orientation.
- B–E
Zoom views on the 40S head‐to‐head contact site (B), the 40S platform‐to‐platform contact site (C), the 40S body‐to‐body contact site (D) and the 60S‐to‐40S contact site (E). In (D), nucleotides missing in the model at the tip of ES6c are indicated by a dashed line.
- F
Location of uS3 (K212) and uS10 (K6 and K8) ubiquitination sites. All ubiquitination sites are in the close spatial vicinity highlighted with red spheres. RACK1 proteins from both the leading and the colliding ribosome form the major head‐to‐head contact bringing together both C‐terminal tails of uS3s and N‐termini of uS10s. For the uS10 ubiquitination sites, only an approximate position is shown as indicated by dashed lines since the uS10 N‐termini containing K6 and K8 are invisible in the structure most likely due to flexibility.

Western blot analysis after overexpression of Hel2 in strains expressing HA‐tagged ribosomal proteins using an anti‐HA antibody. Note the increase in the polyubiquitinated eS7 as well as uS10 and uS3.
Structural overview of the ubiquitination sites on eS7. The general overview on the disome interface is depicted according to Fig 4A. Individual ubiquitination sites are highlighted as red spheres in the detailed cartoon model representation.
Western blot analysis showing the role of Hel2 and Not4 in eS7A ubiquitination: both copies of eS7 (eS7A and eS7B) were deleted and transformed with a plasmid containing HA‐tagged eS7A (eS7‐WT) or eS7A mutated in the four potential ubiquitination sites (4KR). Whole protein extracts we obtained from eS7AΔeS7BΔ cells or cells with an additional deletion in E3 ligases Hel2 and Not4 (hel2Δ and not4Δ). Note that the four mutated lysine residues were responsible for Not4‐dependent monoubiquitination.
Western blot showing that Not4 was required for Hel2‐mediated polyubiquitination of eS7 in Hel2‐bound ribosomal complexes: cells expressing HA‐tagged eS7A or the eS7‐4KR mutant, as well as PTH‐tagged Hel2 in eS7AΔeS7BΔ and eS7AΔeS7BΔnot4Δ background, were probed for eS7‐ubiquitination. Either cell lysates or affinity‐purified Hel2‐ribosome complexes were used. The levels of the ubiquitinated eS7A in the lysates and in the affinity‐purified samples with PTH‐Hel2 were determined by Western blot analysis using an anti‐HA antibody to detect eS7.
Western blotting of in vitro ubiquitination assays of eS7A. The reactions were performed with the indicated components including His‐tagged ubiquitin. Ribosomes were purified from Hel2Δ cells expressing HA‐tagged eS7A. Polyubiquitinated HA‐tagged eS7A (eS7A‐3HA) was detected by Western blot analysis using an anti‐HA antibody. We observed Hel2‐mediated polyubiquitination of the monoubiquitinated eS7A.
Western blot analysis of eS7A in vitro ubiquitination assays showing that Hel2‐mediated polyubiquitination of eS7 was mainly K63‐linked and required Not4‐dependent monoubiquitination: these assays were performed similarly as described in (E) except yeast strains were lacking Not4 (not4Δ). Reactions were performed with the indicated components including His‐tagged ubiquitin and several ubiquitin mutants, and eS7A‐ubiquitination was monitored using an anti‐HA antibody.
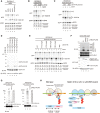
- A–C
Northern blots probing for the 5′ NGD‐IM in mutant cells expressing the R(CGN)12 reporter. (A) 5′ NGD‐IM detection in not4Δski2Δ, hel2Δski2Δ, slh1Δski2Δ and not4Δslh1Δski2Δ. Quantification of full length and the 5′ NGD‐IM relative to the loading control (SCR1) is given below. Note the size difference of 5′ NGD‐IMs resulting from NGDRQC+ and NGDRQC− and that the shorter 5′ NGD intermediate (representing intermediates of the NGDRQC− pathway) was reduced in not4Δslh1Δski2Δ mutant cells. (B) Northern blot as in (A) except uS10‐WTski2Δ or uS10‐K6/8Rski2Δ mutant cells with or without a deletion of Not4 (not4Δ) was used. Note that the shorter 5′ NGD intermediates are also reduced in ski2Δnot4ΔuS10‐K6/8R cells. (C) Cells expressing HA‐tagged eS7A or the eS7A‐4KR mutant in a ski2ΔeS7AΔeS7BΔ or ski2ΔeS7AΔeS7BΔslh1Δ backgrounds were probed for 5′ NGD intermediates by Northern blotting. Note that the four mutated lysine residues in eS7 were responsible for NGDRQC− in the R(CGN)12 reporter.
- D
Western blot analysis showing that K83 and K84 of eS7A are main target sites for Hel2 and Not4‐mediated ubiquitination: similarly, as in Fig 6C, eS7AΔeS7BΔ mutant cells were used and wild‐type and single mutants of HA‐tagged eS7A were expressed. Ubiquitinated eS7‐HA was monitored using an anti‐HA antibody.
- E
Northern Blot analysis probing for the levels of 5′ NGD‐IM derived from the R(CGN)12 reporter expressed in eS7AΔeS7BΔski2Δ cells and HA‐tagged eS7 or eS7 mutants. We observed that ubiquitination at K83 or K84 of eS7A is mainly responsible for NGDRQC−.
- F
The levels of polyubiquitinated eS7A were increased by the overproduction of wild‐type Hel2 (FL) but neither Hel2ΔRING nor Hel2(1–315) mutants. eS7A‐3HAhel2Δ mutant cells harbouring the indicated plasmids expressing wild‐type Hel2 (FL), Hel2ΔRING or Hel2(1–315) mutant proteins were harvested. Protein samples were analysed by Western blotting with an anti‐HA (top panel) or anti‐FLAG antibody (bottom panel).
- G
Hel2(1–315) polyubiquitinates eS7A but not uS10. Western blotting after an in vitro ubiquitination assay using the Hel2(1–315) mutant and purified ribosomes containing HA‐tagged uS10.
- H
Model for quality control pathways induced by R(CGN)12‐mediated translation arrest. If the RQC pathway is intact, the leading ribosome that is stalled by the arrest sequence undergoes RQC. The uS10 ubiquitination and ATPase activity of Slh1/Rqt2‐dependent subunit dissociation induce the endonucleolytic cleavages in the sequence covered by the first and the second ribosome (disome). In the absence of uS10 ubiquitination and Rqt2, RQC in the first ribosome, as well as NGD in the disome, is eliminated. Instead, RQC‐uncoupled NGDRQC− takes place upstream of the disome. The carboxyl‐terminal region of Hel2 is crucial to induce RQC thereby NGD in the disome (NGDRQC+), but dispensable for RQC‐uncoupled NGD (NGDRQC−).

Spot assay showing that severe growth defect of not4∆ and eS7A‐4KR mutant cells irrespective of the presence of anisomycin. Indicated yeast cells were cultured with liquid YPD media, and 10‐fold serial dilutions of the cells were grown on YPD with or without 10 μg/ml anisomycin.
Spot assay showing that not4∆hel2∆ and not4∆slh1∆ exhibited synthetic growth defect. Indicated yeast cells were cultured with liquid YPD media, and then, 10‐fold serial dilutions of the cells were grown on YPD.
Synthetic lethality of xrn1∆not4∆ cells. Spot assay of not4∆ and xrn1∆not4∆ yeast cells harbouring p416GPDp‐FLAG‐NOT4 transformed with p415GPD empty vector or p415GPDp‐FLAG‐NOT4. Transformed cells on the SDC‐Leu medium were cultured with liquid SDC‐Leu media for 1 day, and 10‐fold serial dilutions of the cells were grown on SDC‐Leu‐Ura plate or SDC‐Leu plate with 0.5 g/l 5‐fluoroorotic acid (5‐FOA).
Comment in
-
Ubiquitin-a beacon for all during quality control on the ribosome.EMBO J. 2019 Mar 1;38(5):e101633. doi: 10.15252/embj.2019101633. Epub 2019 Feb 15. EMBO J. 2019. PMID: 30770343 Free PMC article.
Similar articles
-
A distinct mammalian disome collision interface harbors K63-linked polyubiquitination of uS10 to trigger hRQT-mediated subunit dissociation.Nat Commun. 2022 Oct 27;13(1):6411. doi: 10.1038/s41467-022-34097-9. Nat Commun. 2022. PMID: 36302773 Free PMC article.
-
Stalled disomes marked by Hel2-dependent ubiquitin chains undergo Ubp2/Ubp3-mediated deubiquitination upon translational run-off.Commun Biol. 2025 Jan 28;8(1):132. doi: 10.1038/s42003-025-07569-z. Commun Biol. 2025. PMID: 39875504 Free PMC article.
-
Ribosomal collision is not a prerequisite for ZNF598-mediated ribosome ubiquitination and disassembly of ribosomal complexes by ASCC.Nucleic Acids Res. 2024 May 8;52(8):4627-4643. doi: 10.1093/nar/gkae087. Nucleic Acids Res. 2024. PMID: 38366554 Free PMC article.
-
Mechanisms of Translation-coupled Quality Control.J Mol Biol. 2024 Mar 15;436(6):168496. doi: 10.1016/j.jmb.2024.168496. Epub 2024 Feb 15. J Mol Biol. 2024. PMID: 38365086 Review.
-
Proteostasis regulation through ribosome quality control and no-go-decay.Wiley Interdiscip Rev RNA. 2023 Nov-Dec;14(6):e1809. doi: 10.1002/wrna.1809. Epub 2023 Jul 24. Wiley Interdiscip Rev RNA. 2023. PMID: 37488089 Review.
Cited by
-
Readthrough of stop codons under limiting ABCE1 concentration involves frameshifting and inhibits nonsense-mediated mRNA decay.Nucleic Acids Res. 2020 Oct 9;48(18):10259-10279. doi: 10.1093/nar/gkaa758. Nucleic Acids Res. 2020. PMID: 32941650 Free PMC article.
-
Structural basis for clearing of ribosome collisions by the RQT complex.Nat Commun. 2023 Feb 17;14(1):921. doi: 10.1038/s41467-023-36230-8. Nat Commun. 2023. PMID: 36801861 Free PMC article.
-
Ribosome-associated quality-control mechanisms from bacteria to humans.Mol Cell. 2022 Apr 21;82(8):1451-1466. doi: 10.1016/j.molcel.2022.03.038. Mol Cell. 2022. PMID: 35452614 Free PMC article. Review.
-
Combinations of slow-translating codon clusters can increase mRNA half-life in Saccharomyces cerevisiae.Proc Natl Acad Sci U S A. 2021 Dec 21;118(51):e2026362118. doi: 10.1073/pnas.2026362118. Proc Natl Acad Sci U S A. 2021. PMID: 34911752 Free PMC article.
-
Znf598-mediated Rps10/eS10 ubiquitination contributes to the ribosome ubiquitination dynamics during zebrafish development.RNA. 2023 Dec;29(12):1910-1927. doi: 10.1261/rna.079633.123. Epub 2023 Sep 26. RNA. 2023. PMID: 37751929 Free PMC article.
References
-
- Becker T, Franckenberg S, Wickles S, Shoemaker CJ, Anger AM, Armache JP, Sieber H, Ungewickell C, Berninghausen O, Daberkow I, Karcher A, Thomm M, Hopfner KP, Green R, Beckmann R (2012) Structural basis of highly conserved ribosome recycling in eukaryotes and archaea. Nature 482: 501–506 - PMC - PubMed
-
- Beckmann R, Spahn CM, Eswar N, Helmers J, Penczek PA, Sali A, Frank J, Blobel G (2001) Architecture of the protein‐conducting channel associated with the translating 80S ribosome. Cell 107: 361–372 - PubMed
-
- Ben‐Shem A, Jenner L, Yusupova G, Yusupov M (2010) Crystal structure of the eukaryotic ribosome. Science 330: 1203–1209 - PubMed
-
- Bosanac I, Wertz IE, Pan B, Yu C, Kusam S, Lam C, Phu L, Phung Q, Maurer B, Arnott D, Kirkpatrick DS, Dixit VM, Hymowitz SG (2010) Ubiquitin binding to A20 ZnF4 is required for modulation of NF‐kappaB signaling. Mol Cell 40: 548–557 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

