An alternative N-terminal fold of the intestine-specific annexin A13a induces dimerization and regulates membrane-binding
- PMID: 30610115
- PMCID: PMC6416438
- DOI: 10.1074/jbc.RA118.004571
An alternative N-terminal fold of the intestine-specific annexin A13a induces dimerization and regulates membrane-binding
Abstract
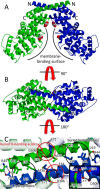
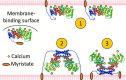
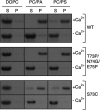
Annexin proteins function as Ca2+-dependent regulators of membrane trafficking and repair that may also modulate membrane curvature. Here, using high-resolution confocal imaging, we report that the intestine-specific annexin A13 (ANX A13) localizes to the tips of intestinal microvilli and determined the crystal structure of the ANX A13a isoform to 2.6 Å resolution. The structure revealed that the N terminus exhibits an alternative fold that converts the first two helices and the associated helix-loop-helix motif into a continuous α-helix, as stabilized by a domain-swapped dimer. We also found that the dimer is present in solution and partially occludes the membrane-binding surfaces of annexin, suggesting that dimerization may function as a means for regulating membrane binding. Accordingly, as revealed by in vitro binding and cellular localization assays, ANX A13a variants that favor a monomeric state exhibited increased membrane association relative to variants that favor the dimeric form. Together, our findings support a mechanism for how the association of the ANX A13a isoform with the membrane is regulated.
Keywords: annexin; calcium regulation; intestinal microvilli; membrane curvature; membrane fusion; oligomerization; protein folding; protein structure; structure-function.
© 2019 McCulloch et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Creutz C. E., Pazoles C. J., and Pollard H. B. (1978) Identification and purification of an adrenal medullary protein (synexin) that causes calcium-dependent aggregation of isolated chromaffin granules. J. Biol. Chem. 253, 2858–2866 - PubMed
-
- Huber R., Berendes R., Burger A., Luecke H., and Karshikov A. (1992) Annexin V-crystal structure and its implications on function. Behring Inst. Mitt. 107–125 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

