A Remote cis-Regulatory Region Is Required for NIN Expression in the Pericycle to Initiate Nodule Primordium Formation in Medicago truncatula
- PMID: 30610167
- PMCID: PMC6391699
- DOI: 10.1105/tpc.18.00478
A Remote cis-Regulatory Region Is Required for NIN Expression in the Pericycle to Initiate Nodule Primordium Formation in Medicago truncatula
Abstract
The legume-rhizobium symbiosis results in nitrogen-fixing root nodules, and their formation involves both intracellular infection initiated in the epidermis and nodule organogenesis initiated in inner root cell layers. NODULE INCEPTION (NIN) is a nodule-specific transcription factor essential for both processes. These NIN-regulated processes occur at different times and locations in the root, demonstrating a complex pattern of spatiotemporal regulation. We show that regulatory sequences sufficient for the epidermal infection process are located within a 5 kb region directly upstream of the NIN start codon in Medicago truncatula Furthermore, we identify a remote upstream cis-regulatory region required for the expression of NIN in the pericycle, and we show that this region is essential for nodule organogenesis. This region contains putative cytokinin response elements and is conserved in eight more legume species. Both the cytokinin receptor 1, which is essential for nodule primordium formation, and the B-type response regulator RR1 are expressed in the pericycle in the susceptible zone of the uninoculated root. This, together with the identification of the cytokinin-responsive elements in the NIN promoter, strongly suggests that NIN expression is initially triggered by cytokinin signaling in the pericycle to initiate nodule primordium formation.
© 2019 American Society of Plant Biologists. All rights reserved.
Figures




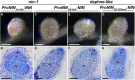




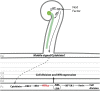
Comment in
-
Solving a Cold Case: Identification of Promoter Elements to Complement Medicago nin Mutants.Plant Cell. 2019 Jan;31(1):7-8. doi: 10.1105/tpc.19.00019. Epub 2019 Jan 16. Plant Cell. 2019. PMID: 30651346 Free PMC article. No abstract available.
Similar articles
-
The NIN Transcription Factor Coordinates Diverse Nodulation Programs in Different Tissues of the Medicago truncatula Root.Plant Cell. 2015 Dec;27(12):3410-24. doi: 10.1105/tpc.15.00461. Epub 2015 Dec 15. Plant Cell. 2015. PMID: 26672071 Free PMC article.
-
NODULE INCEPTION Recruits the Lateral Root Developmental Program for Symbiotic Nodule Organogenesis in Medicago truncatula.Curr Biol. 2019 Nov 4;29(21):3657-3668.e5. doi: 10.1016/j.cub.2019.09.005. Epub 2019 Sep 19. Curr Biol. 2019. PMID: 31543454 Free PMC article.
-
Spatiotemporal cytokinin response imaging and ISOPENTENYLTRANSFERASE 3 function in Medicago nodule development.Plant Physiol. 2022 Jan 20;188(1):560-575. doi: 10.1093/plphys/kiab447. Plant Physiol. 2022. PMID: 34599592 Free PMC article.
-
Evolution of NIN and NIN-like Genes in Relation to Nodule Symbiosis.Genes (Basel). 2020 Jul 11;11(7):777. doi: 10.3390/genes11070777. Genes (Basel). 2020. PMID: 32664480 Free PMC article. Review.
-
Gene Expression in Nitrogen-Fixing Symbiotic Nodule Cells in Medicago truncatula and Other Nodulating Plants.Plant Cell. 2020 Jan;32(1):42-68. doi: 10.1105/tpc.19.00494. Epub 2019 Nov 11. Plant Cell. 2020. PMID: 31712407 Free PMC article. Review.
Cited by
-
Auxin methylation by IAMT1, duplicated in the legume lineage, promotes root nodule development in Lotus japonicus.Proc Natl Acad Sci U S A. 2022 Mar 8;119(10):e2116549119. doi: 10.1073/pnas.2116549119. Epub 2022 Mar 2. Proc Natl Acad Sci U S A. 2022. PMID: 35235457 Free PMC article.
-
An LCO-responsive homolog of NODULE INCEPTION positively regulates lateral root formation in Populus sp.Plant Physiol. 2022 Oct 27;190(3):1699-1714. doi: 10.1093/plphys/kiac356. Plant Physiol. 2022. PMID: 35929094 Free PMC article.
-
Functional and comparative genomics reveals conserved noncoding sequences in the nitrogen-fixing clade.New Phytol. 2022 Apr;234(2):634-649. doi: 10.1111/nph.18006. Epub 2022 Feb 21. New Phytol. 2022. PMID: 35092309 Free PMC article.
-
GmTOC1b inhibits nodulation by repressing GmNIN2a and GmENOD40-1 in soybean.Front Plant Sci. 2022 Nov 11;13:1052017. doi: 10.3389/fpls.2022.1052017. eCollection 2022. Front Plant Sci. 2022. PMID: 36438085 Free PMC article.
-
Celebrating 20 Years of Genetic Discoveries in Legume Nodulation and Symbiotic Nitrogen Fixation.Plant Cell. 2020 Jan;32(1):15-41. doi: 10.1105/tpc.19.00279. Epub 2019 Oct 24. Plant Cell. 2020. PMID: 31649123 Free PMC article. Review.
References
-
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. (1990). Basic local alignment search tool. J. Mol. Biol. 215: 403–410. - PubMed
-
- Benfey P.N., Chua N.-H. (1990). The cauliflower mosaic virus 35S promoter: Combinatorial regulation of transcription in plants. Science 250: 959–966. - PubMed
-
- Bertioli D.J., et al. (2016). The genome sequences of Arachis duranensis and Arachis ipaensis, the diploid ancestors of cultivated peanut. Nat. Genet. 48: 438–446. - PubMed
-
- Boivin S., Kazmierczak T., Brault M., Wen J., Gamas P., Mysore K.S., Frugier F. (2016). Different cytokinin histidine kinase receptors regulate nodule initiation as well as later nodule developmental stages in Medicago truncatula. Plant Cell Environ. 39: 2198–2209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

