Aligned hydrogel tubes guide regeneration following spinal cord injury
- PMID: 30610918
- PMCID: PMC6369008
- DOI: 10.1016/j.actbio.2018.12.052
Aligned hydrogel tubes guide regeneration following spinal cord injury
Abstract
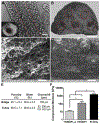
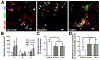
Directing the organization of cells into a tissue with defined architectures is one use of biomaterials for regenerative medicine. To this end, hydrogels are widely investigated as they have mechanical properties similar to native soft tissues and can be formed in situ to conform to a defect. Herein, we describe the development of porous hydrogel tubes fabricated through a two-step polymerization process with an intermediate microsphere phase that provides macroscale porosity (66.5%) for cell infiltration. These tubes were investigated in a spinal cord injury model, with the tubes assembled to conform to the injury and to provide an orientation that guides axons through the injury. Implanted tubes had good apposition and were integrated with the host tissue due to cell infiltration, with a transient increase in immune cell infiltration at 1 week that resolved by 2 weeks post injury compared to a gelfoam control. The glial scar was significantly reduced relative to control, which enabled robust axon growth along the inner and outer surface of the tubes. Axon density within the hydrogel tubes (1744 axons/mm2) was significantly increased more than 3-fold compared to the control (456 axons/mm2), with approximately 30% of axons within the tube myelinated. Furthermore, implantation of hydrogel tubes enhanced functional recovery relative to control. This modular assembly of porous tubes to fill a defect and directionally orient tissue growth could be extended beyond spinal cord injury to other tissues, such as vascular or musculoskeletal tissue. STATEMENT OF SIGNIFICANCE: Tissue engineering approaches that mimic the native architecture of healthy tissue are needed following injury. Traditionally, pre-molded scaffolds have been implemented but require a priori knowledge of wound geometries. Conversely, hydrogels can conform to any injury, but do not guide bi-directional regeneration. In this work, we investigate the feasibility of a system of modular hydrogel tubes to promote bi-directional regeneration after spinal cord injury. This system allows for tubes to be cut to size during surgery and implanted one-by-one to fill any injury, while providing bi-directional guidance. Moreover, this system of tubes can be broadly applied to tissue engineering approaches that require a modular guidance system, such as repair to vascular or musculoskeletal tissues.
Keywords: Axon elongation; Modular biomaterial; Spinal cord injury; Tissue repair.
Copyright © 2019 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
The authors have no competing interests to declare.
Figures
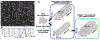
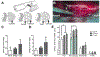
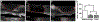


References
-
- Xu T, Zhao W, Zhu JM, Albanna MZ, Yoo JJ, Atala A, Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology, Biomaterials 34(1) (2013) 130–9. - PubMed
-
- Mapili G, Lu Y, Chen S, Roy K, Laser-layered microfabrication of spatially patterned functionalized tissue-engineering scaffolds, J Biomed Mater Res B Appl Biomater 75(2) (2005) 414–24. - PubMed
-
- Raineteau O, Schwab ME, Plasticity of motor systems after incomplete spinal cord injury, Nat Rev Neurosci 2(4) (2001) 263–73. - PubMed
-
- Anderson KD, Cowan RE, Horsewell J, Facilitators and Barriers to Spinal Cord Injury Clinical Trial Participation: Multi-National Perspective of People Living with Spinal Cord Injury, J Neurotrauma 33(5) (2016) 493–9. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC, Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection, Exp Neurol 139(2) (1996) 244–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

