Transplantation of mesenchymal stem cells genetically engineered to overexpress interleukin-10 promotes alternative inflammatory response in rat model of traumatic brain injury
- PMID: 30611291
- PMCID: PMC6320578
- DOI: 10.1186/s12974-018-1383-2
Transplantation of mesenchymal stem cells genetically engineered to overexpress interleukin-10 promotes alternative inflammatory response in rat model of traumatic brain injury
Erratum in
-
Correction to: Transplantation of mesenchymal stem cells genetically engineered to overexpress interleukin-10 promotes alternative inflammatory response in rat model of traumatic brain injury.J Neuroinflammation. 2022 Jan 11;19(1):15. doi: 10.1186/s12974-021-02362-1. J Neuroinflammation. 2022. PMID: 35016685 Free PMC article. No abstract available.
Abstract
Background: Traumatic brain injury (TBI) is a major cause for long-term disability, yet the treatments available that improve outcomes after TBI limited. Neuroinflammatory responses are key contributors to determining patient outcomes after TBI. Transplantation of mesenchymal stem cells (MSCs), which release trophic and pro-repair cytokines, represents an effective strategy to reduce inflammation after TBI. One such pro-repair cytokine is interleukin-10 (IL-10), which reduces pro-inflammatory markers and trigger alternative inflammatory markers, such as CD163. In this study, we tested the therapeutic effects of MSCs that were engineered to overexpress IL-10 when transplanted into rats following TBI in the medial frontal cortex.
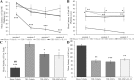
Methods: Thirty-six hours following TBI, rats were transplanted with MSCs and then assessed for 3 weeks on a battery of behavioral tests that measured motor and cognitive abilities. Histological evaluation was then done to measure the activation of the inflammatory response. Additionally, immunomodulatory effects were evaluated by immunohistochemistry and Western blot analyses.
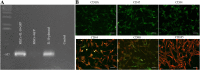
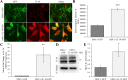
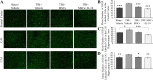
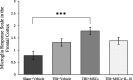
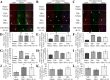
Results: A significant improvement in fine motor function was observed in rats that received transplants of MSCs engineered to overexpress IL-10 (MSCs + IL-10) or MSCs alone compared to TBI + vehicle-treated rats. Although tissue spared was unchanged, anti-inflammatory effects were revealed by a reduction in the number of glial fibrillary acidic protein cells and CD86 cells in both TBI + MSCs + IL-10 and TBI + MSC groups compared to TBI + vehicle rats. Microglial activation was significantly increased in the TBI + MSC group when compared to the sham + vehicle group. Western blot data suggested a reduction in tumor necrosis factor-alpha in the TBI + MSCs + IL-10 group compared to TBI + MSC group. Immunomodulatory effects were demonstrated by a shift from classical inflammation expression (CD86) to an alternative inflammation state (CD163) in both treatments with MSCs and MSCs + IL-10. Furthermore, co-labeling of both CD86 and CD163 was detected in the same cells, suggesting a temporal change in macrophage expression.
Conclusions: Overall, our findings suggest that transplantation of MSCs that were engineered to overexpress IL-10 can improve functional outcomes by providing a beneficial perilesion environment. This improvement may be explained by the shifting of macrophage expression to a more pro-repair state, thereby providing a possible new therapy for treating TBI.
Keywords: CD163; Interleukin-10; Mesenchymal stem cells; Neuroinflammation; Traumatic brain injury.
Conflict of interest statement
Ethics approval and consent to participate
All animal procedures were approved by the Institutional Animal Care and Use Committee at Central Michigan University (#16-11). Viral construction was approved by the Institutional Biosafety Committee at Central Michigan University.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

