Disease-Modifying Therapies in Type 1 Diabetes: A Look into the Future of Diabetes Practice
- PMID: 30612319
- PMCID: PMC6613813
- DOI: 10.1007/s40265-018-1035-y
Disease-Modifying Therapies in Type 1 Diabetes: A Look into the Future of Diabetes Practice
Abstract
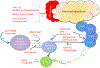
The novel understanding that the presence of multiple islet autoantibodies, indicating islet autoimmunity, inevitably leads to type 1 diabetes mellitus (T1DM) has necessitated the development of a new staging classification system for the condition. Coupled with an improved understanding of the disease course, the realization that T1DM appears to be more heterogeneous than previously thought has led to unique opportunities to develop more targeted therapies that may be applied even before the onset of dysglycemia or symptoms. To date, several therapies have been trialed to delay or halt disease progression in both presymptomatic and clinical T1DM, each demonstrating varying degrees of effectiveness, toxicity, and utility. Key research supports the eventual implementation of immunotherapy in autoimmune diabetes, potentially calling for a paradigm shift among care providers. It will likely be necessary to develop new approaches to trial design and to address potential barriers to progress before an effective treatment for the disease may be achieved.
Conflict of interest statement
Conflict of Interest
Carla Greenbaum reports personal fees from Lilly, grants and personal fees from NovoNordisk, personal fees from Bristol Myers Squibb, grants and personal fees from Janssen, and personal fees from Pfizer.
Sandra Lord and Dana VanBuecken declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Figures




References
-
- Walsh D and McWilliams D, Mechanisms, impact and management of pain in rheumatoid arthritis. Nat Rev Rheumatol, 2014. 10: p. 581–592. - PubMed
-
- Firestein G, Ravinder N, and Romain P, Pathogenesis of rheumatoid arthritis. 2017, UpToDate.
-
- group D, The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N. Engl. J. Med, 1993. 329(14): p. 977–986. - PubMed
-
- Secrest AM, Washington RE, and Orchard TJ, Mortality in Type 1 DIabetes, in Diabetes in America, 3rd Edition 2016, National Institutes of Health; p. 1–16.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

