Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development
- PMID: 30612902
- PMCID: PMC6345627
- DOI: 10.1016/j.cub.2018.11.059
Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development
Abstract
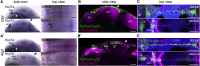
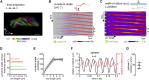
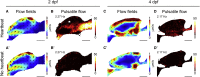
Motile cilia are miniature, propeller-like extensions, emanating from many cell types across the body. Their coordinated beating generates a directional fluid flow, which is essential for various biological processes, from respiration to reproduction. In the nervous system, ependymal cells extend their motile cilia into the brain ventricles and contribute to cerebrospinal fluid (CSF) flow. Although motile cilia are not the only contributors to CSF flow, their functioning is crucial, as patients with motile cilia defects develop clinical features, like hydrocephalus and scoliosis. CSF flow was suggested to primarily deliver nutrients and remove waste, but recent studies emphasized its role in brain development and function. Nevertheless, it remains poorly understood how ciliary beating generates and organizes CSF flow to fulfill these roles. Here, we study motile cilia and CSF flow in the brain ventricles of larval zebrafish. We identified that different populations of motile ciliated cells are spatially organized and generate a directional CSF flow powered by ciliary beating. Our investigations revealed that CSF flow is confined within individual ventricular cavities, with little exchange of fluid between ventricles, despite a pulsatile CSF displacement caused by the heartbeat. Interestingly, our results showed that the ventricular boundaries supporting this compartmentalized CSF flow are abolished during bodily movement, highlighting that multiple physiological processes regulate the hydrodynamics of CSF flow. Finally, we showed that perturbing cilia reduces hydrodynamic coupling between the brain ventricles and disrupts ventricular development. We propose that motile-cilia-generated flow is crucial in regulating the distribution of CSF within and across brain ventricles.
Keywords: brain; brain ventricle; cerebrospinal fluid; cilia; ependymal cells; flow; fluid dynamics; foxj1; heartbeat; zebrafish.
Copyright © 2018 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures




References
-
- Fliegauf M., Benzing T., Omran H. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 2007;8:880–893. - PubMed
-
- Mitchison H.M., Valente E.M. Motile and non-motile cilia in human pathology: from function to phenotypes. J. Pathol. 2017;241:294–309. - PubMed
-
- Sampaio P., Ferreira R.R., Guerrero A., Pintado P., Tavares B., Amaro J., Smith A.A., Montenegro-Johnson T., Smith D.J., Lopes S.S. Left-right organizer flow dynamics: how much cilia activity reliably yields laterality? Dev. Cell. 2014;29:716–728. - PubMed
-
- Nonaka S., Tanaka Y., Okada Y., Takeda S., Harada A., Kanai Y., Kido M., Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

