[Acute and chronic toxicity of 0.5% podophyllotoxin-loaded nanostructured lipid carriers to vaginal mucosa in rabbits and rats]
- PMID: 30613025
- PMCID: PMC6744213
- DOI: 10.12122/j.issn.1673-4254.2018.12.21
[Acute and chronic toxicity of 0.5% podophyllotoxin-loaded nanostructured lipid carriers to vaginal mucosa in rabbits and rats]
Abstract
Objective: To test the acute and chronic toxicity of topical application of 0.5% podophyllotoxin-loaded nanostructured lipid carriers (POD-NLC) to the vaginal mucosa.
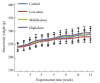
Methods: Twelve New Zealand rabbits were randomized into 3 groups and subjected to daily topical applications of normal saline (control group), 0.5% podophyllotoxin tincture (POD-T) or 0.5% POD-NLC on the vaginal mucosa for 10 consecutive days, and the pathological changes in the mucosa were graded using the Eckstein scoring system.The acute toxicity of POD-NLC was tested in 20 SD female rats, which received intravaginal administration of POD-NLC or vehicle for 3 times within 24 h; After 14 days of continuous observation, the rats were dissected for calculating the viscera coefficient.For testing the chronic toxicity of POD-NLC, 80 SD female rats were randomized into 4 groups and subjected to daily intravaginal administration of the vehicle or POD-NLC at low, moderate or high doses for 13 consecutive weeks.The rats were weighed once a week and at the end of the experiment, 2/3 of the rats from each group were sacrificed to collect blood samples, calculate the viscera coefficient, and examine the pathological changes in the liver.The remaining 1/3 rats were observed for another 2 weeks without further drug treatment and the same examinations were performed.
Results: In the rabbits, 0.5% POD-NLC elicited only mild irritation while POD-T caused moderate irritation of the vaginal mucosa.In the acute toxicity test, the organ coefficients were comparable between the rats treated with the vehicle and POD-NLC (P>0.05).Long-term intravaginal administration of POD-NLC did not produce significant changes in the behavior, activity, body weight, blood biochemical profiles or organ coefficient as compared with the vehicle control group (P>0.05).
Conclusions: Intravaginal administration of 0.5% POD-NLC causes very mild irritation without obvious acute or chronic toxicity to the vaginal mucosa in rabbits and rats.
目的: 考察0.5%鬼臼毒素纳米脂质载体在阴道黏膜的安全性。
方法: (1) 刺激性试验:将12只新西兰兔均分为3组, 分别予生理盐水(NS)、0.5%鬼臼毒素酊剂(POD-T)和0.5%鬼臼毒素纳米脂质载体(POD-NLC), 阴道给药10 d后取阴道组织行病理检查, 根据Eckstein评分标准进行评估。(2)急性毒性试验:将20只SD雌性大鼠均分2组, 分别予赋形剂和POD-NLC, 以300倍临床剂量在24 h内分3次阴道给药, 连续观察14 d后解剖并计算脏器系数。(3)长期毒性试验:80只SD雌性大鼠均分4组, 分别予赋形剂、低剂量POD-NLC (15倍临床剂量)、中剂量POD-NLC (30倍临床剂量)和高剂量POD-NLC (60倍临床剂量)。连续13周每日阴道给药1次, 每周记录体质量, 给药结束后对每组2/3的大鼠进行抽血化验、解剖并计算脏器系数和肝脏组织病理学检查。为观察药物延迟毒性, 每组剩余1/3的大鼠停药观察2周后做同前处理。
结果: (1) 刺激性试验:0.5% POD-NLC对阴道黏膜为极轻刺激性, 0.5% POD-T为中度刺激性; (2)急性毒性试验:赋形剂组与POD-NLC组大鼠相比, 各器官的脏器系数的差异无统计学意义(P>0.05);(3)长期毒性试验:赋形剂组与POD-NLC各剂量组大鼠的一般表现、行为活动、体质量变化、血常规、血生化、重要器官脏器系数及肝脏病理未见明显异常, 且组间差异无统计学意义(P>0.05)。
结论: POD-NLC在阴道黏膜用药刺激性较小, 对机体无明显的急性及长期毒性, 有较高的安全性。
Keywords: acute toxicity; chronic toxicity; condyloma acuminate; irritation; nanostructured lipid carrier; podophyllotoxin.
Figures


Similar articles
-
[Distribution of podophyllotoxin-loaded nanostructured lipid carriers after topical application on cervical mucosa in Tibet minipigs].Nan Fang Yi Ke Da Xue Xue Bao. 2016 Aug 20;36(9):1237-1241. Nan Fang Yi Ke Da Xue Xue Bao. 2016. PMID: 27687657 Chinese.
-
Podophyllotoxin-Loaded Nanostructured Lipid Carriers for Skin Targeting: In Vitro and In Vivo Studies.Molecules. 2016 Nov 17;21(11):1549. doi: 10.3390/molecules21111549. Molecules. 2016. PMID: 27869698 Free PMC article.
-
[Preparation of podophyllotoxin nanostructured lipid carriers and its effects on immortalized human cervical epithelial cells with HPV infection in vitro].Nan Fang Yi Ke Da Xue Xue Bao. 2011 Jun;31(6):1023-8. Nan Fang Yi Ke Da Xue Xue Bao. 2011. PMID: 21690061 Chinese.
-
Condyloma eradication: self-therapy with 0.15-0.5% podophyllotoxin versus 20-25% podophyllin preparations--an integrated safety assessment.Regul Toxicol Pharmacol. 2001 Apr;33(2):117-37. doi: 10.1006/rtph.2000.1446. Regul Toxicol Pharmacol. 2001. PMID: 11350195 Review.
-
Final report of the safety assessment of Alcohol Denat., including SD Alcohol 3-A, SD Alcohol 30, SD Alcohol 39, SD Alcohol 39-B, SD Alcohol 39-C, SD Alcohol 40, SD Alcohol 40-B, and SD Alcohol 40-C, and the denaturants, Quassin, Brucine Sulfate/Brucine, and Denatonium Benzoate.Int J Toxicol. 2008;27 Suppl 1:1-43. doi: 10.1080/10915810802032388. Int J Toxicol. 2008. PMID: 18569160 Review.
References
-
- Zhang Y, Jiang S, Lin H, et al. Application of dermoscopy image analysis technique in diagnosing urethral condylomata acuminata. http://europepmc.org/abstract/MED/29641700. An Bras Dermatol. 2018;93(1):67–71. [Zhang Y, Jiang S, Lin H, et al.Application of dermoscopy image analysis technique in diagnosing urethral condylomata acuminata [J]. An Bras Dermatol, 2018, 93(1):67-71.] - PMC - PubMed
-
- Nelson E L, Bogliatto F, Stockdale C K. Vulvar intraepithelial neoplasia (VIN) and vondylomata. Clin Obstet Gynecol. 2015;58(3):512–25. doi: 10.1097/GRF.0000000000000132. [Nelson E L, Bogliatto F, Stockdale C K.Vulvar intraepithelial neoplasia (VIN) and vondylomata[J]. Clin Obstet Gynecol, 2015, 58 (3):512-25.] - DOI - PubMed
-
- Thurgar E, Barton S, Karner C, et al. Clinical effectiveness and costeffectiveness of interventions for the treatment of anogenital warts: systematic review and economic evaluation. Health Technol Assess. 2016;20(24):1–486. doi: 10.3310/hta20240. [Thurgar E, Barton S, Karner C, et al.Clinical effectiveness and costeffectiveness of interventions for the treatment of anogenital warts: systematic review and economic evaluation[J]. Health Technol Assess, 2016, 20(24):1-486.] - DOI - PMC - PubMed
-
- Hu L, Zhou X, Zhang H, et al. Exposure to podophyllotoxin inhibits oocyte meiosis by disturbing meiotic spindle formation. Sci Rep. 2018;8(1):10145. doi: 10.1038/s41598-018-28544-1. [Hu L, Zhou X, Zhang H, et al.Exposure to podophyllotoxin inhibits oocyte meiosis by disturbing meiotic spindle formation[J]. Sci Rep, 2018, 8(1):10145.] - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
