Chemoresistance Transmission via Exosome-Mediated EphA2 Transfer in Pancreatic Cancer
- PMID: 30613276
- PMCID: PMC6299429
- DOI: 10.7150/thno.26650
Chemoresistance Transmission via Exosome-Mediated EphA2 Transfer in Pancreatic Cancer
Abstract
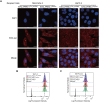
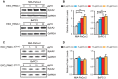
Rationale: Exosomes are small extracellular vesicles secreted by most cells that are found in blood and other bodily fluids, and which contain cytoplasmic material and membrane factors corresponding to their cell type of origin. Exosome membrane factors and contents have been reported to alter adjacent and distant cell behavior in multiple studies, but the impact of cancer-derived exosomes on chemoresistance is less clear. Methods: Exosomes isolated from three pancreatic cancer (PC) cell lines displaying variable gemcitabine (GEM) resistance (PANC-1, MIA PaCa-2, and BxPC-3) were tested for their capacity to transmit chemoresistance among these cell lines. Comparative proteomics was performed to identify key exosomal proteins that conferred chemoresistance. Cell survival was assessed in GEM responsive PC cell lines treated with recombinant Ephrin type-A receptor 2 (EphA2), a candidate chemoresistance transfer factor, or exosomes from a chemoresistant PC cell line treated with or without EphA2 shRNA. Results: Exosomes from chemoresistant PANC-1 cells increased the GEM resistance of MIA PaCa-2 and BxPC-3 cell cultures. Comparative proteomics determined that PANC-1 exosomes overexpressed Ephrin type-A receptor 2 (EphA2) versus exosomes of less chemoresistant PC cell lines MIA PaCa-2 and BxPC-3. EphA2-knockdown in PANC-1 cells inhibited their ability to transmit exosome-mediated chemoresistance to MIA PaCa-2 and BxPC-3, while treatment of MIA PaCa-2 and BxPC-3 cells with soluble EphA2 did not promote chemoresistance, indicating that membrane carried EphA2 was important for the EphA2 chemoresistance effect. Conclusion: Exosomal EphA2 expression could transmit chemoresistance and may potentially serve as a minimally-invasive predictive biomarker for PC treatment response. Further work should address whether additional exosomal factors regulate resistance to other cancer therapeutic agents for PC or other cancer types.
Keywords: Cytotoxic resistance; EphA2; Exosome; Gemcitabine; Pancreatic cancer.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–54. - PubMed
-
- Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR. et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. - PubMed
-
- Ryan DP. Chemotherapy for advanced exocrine pancreatic cancer. UpToDate; 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

