Dendrimer- and copolymer-based nanoparticles for magnetic resonance cancer theranostics
- PMID: 30613300
- PMCID: PMC6299700
- DOI: 10.7150/thno.27828
Dendrimer- and copolymer-based nanoparticles for magnetic resonance cancer theranostics
Abstract
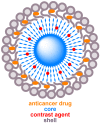
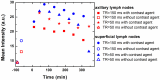
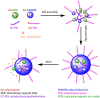
Cancer theranostics is one of the most important approaches for detecting and treating patients at an early stage. To develop such a technique, accurate detection, specific targeting, and controlled delivery are the key components. Various kinds of nanoparticles have been proposed and demonstrated as potential nanovehicles for cancer theranostics. Among them, polymer-like dendrimers and copolymer-based core-shell nanoparticles could potentially be the best possible choices. At present, magnetic resonance imaging (MRI) is widely used for clinical purposes and is generally considered the most convenient and noninvasive imaging modality. Superparamagnetic iron oxide (SPIO) and gadolinium (Gd)-based dendrimers are the major nanostructures that are currently being investigated as nanovehicles for cancer theranostics using MRI. These structures are capable of specific targeting of tumors as well as controlled drug or gene delivery to tumor sites using pH, temperature, or alternating magnetic field (AMF)-controlled mechanisms. Recently, Gd-based pseudo-porous polymer-dendrimer supramolecular nanoparticles have shown 4-fold higher T1 relaxivity along with highly efficient AMF-guided drug release properties. Core-shell copolymer-based nanovehicles are an equally attractive alternative for designing contrast agents and for delivering anti-cancer drugs. Various copolymer materials could be used as core and shell components to provide biostability, modifiable surface properties, and even adjustable imaging contrast enhancement. Recent advances and challenges in MRI cancer theranostics using dendrimer- and copolymer-based nanovehicles have been summarized in this review article, along with new unpublished research results from our laboratories.
Keywords: cancer theranostics; copolymer nanoparticle; dendrimer nanoparticle; magnetic resonance.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Sunderland CJ, Steiert M, Talmadge JE. et al. Targeted nanoparticles for detecting and treating cancer. Drug Dev Res. 2006;67:70–93.
-
- Kelkar SS, Reineke TM. Theranostics: Combining imaging and therapy. Bioconjug Chem. 2011;22:1879–1903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

