Analogues of the Lignan Pinoresinol as Novel Lead Compounds for P-glycoprotein (P-gp) Inhibitors
- PMID: 30613324
- PMCID: PMC6295845
- DOI: 10.1021/acsmedchemlett.8b00324
Analogues of the Lignan Pinoresinol as Novel Lead Compounds for P-glycoprotein (P-gp) Inhibitors
Abstract
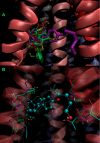
To find novel P-gp-inhibitors, a library of pregnane X receptor (PXR) ligands and the ZINC DrugsNow library were superimposed on the P-gp inhibitor (+)-pinoresinol (1) used as a query for a three-dimensional similarity search. After determining the TanimotoCombo index of similarity with 1, eight compounds from the PXR library and two ZINC compounds were selected for biological evaluation. The P-gp inhibition study showed that compounds 7, 8, and 9 successfully increased intracellular doxorubicin (DOX) accumulation in the P-gp overexpressed Lucena 1 cells from 25, 12.5, and 6.25 μM, respectively. Among a series of analogues of 9, compounds 26-30 were shown to be active, with 26 and 27 causing a significant increase in DOX accumulation from 1.56 μM and rendering Lucena 1 sensitive to DOX from 1.56 and 0.78 μM, respectively. Molecular modeling studies showed that both compounds bind to the P-gp at transmembrane helices (TMH) 4, 5, and 6, with 27 also showing contacts with TMH 3.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- González M. L.; Vera D. M. A.; Laiolo J.; Joray M. B.; Maccioni M.; Palacios S. M.; Molina G.; Lanza P. A.; Gancedo S.; Rumjanek V.; Carpinella M. C. Mechanism Underlying the Reversal of Drug Resistance in P-Glycoprotein-Expressing Leukemia Cells by Pinoresinol and the Study of a Derivative. Front. Pharmacol. 2017, 8, x.10.3389/fphar.2017.00205. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

