Oscillations and Spike Entrainment
- PMID: 30613382
- PMCID: PMC6305216
- DOI: 10.12688/f1000research.16451.1
Oscillations and Spike Entrainment
Abstract
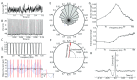
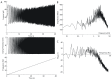
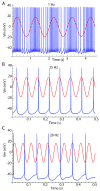
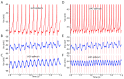
Oscillatory input to networks, as indicated by field potentials, must entrain neuronal firing to be a causal agent in brain activity. Even when the oscillatory input is prominent, entrainment of firing is not a foregone conclusion but depends on the intrinsic dynamics of the postsynaptic neurons, including cell type-specific resonances, and background firing rates. Within any local network of neurons, only a subset of neurons may have their firing entrained by an oscillating synaptic input, and oscillations of different frequency may engage separate subsets of neurons.
Keywords: LFP; oscillation; phase-locking.
Conflict of interest statement
No competing interests were disclosed.No competing interests were disclosed.No competing interests were disclosed.
Figures
References
-
- Buzsáki G: Rhythms of the Brain. Oxford University Press;2006. 10.1093/acprof:oso/9780195301069.001.0001 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

