MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection
- PMID: 30613778
- PMCID: PMC6314830
- DOI: 10.1126/sciadv.aav0216
MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection
Abstract
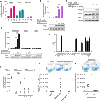
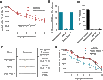
Mast cells (MCs) are strategically distributed at barrier sites and prestore various immunocyte-recruiting cytokines, making them ideal targets for selective activation to treat peripheral infections. Here, we report that topical treatment with mastoparan, a peptide MC activator (MCA), enhances clearance of Staphylococcus aureus from infected mouse skins and accelerates healing of dermonecrotic lesions. Mastoparan functions by activating connective tissue MCs (CTMCs) via the MRGPRX2 (Mas-related G protein-coupled receptor member X2) receptor. Peripheral CTMC activation, in turn, enhances recruitment of bacteria-clearing neutrophils and wound-healing CD301b+ dendritic cells. Consistent with MCs playing a master coordinating role, MC activation also augmented migration of various antigen-presenting dendritic cells to draining lymph nodes, leading to stronger protection against a second infection challenge. MCAs therefore orchestrate both the innate and adaptive immune arms, which could potentially be applied to combat peripheral infections by a broad range of pathogens.
Figures




References
-
- Marshall J. S., Mast-cell responses to pathogens. Nat. Rev. Immunol. 4, 787–799 (2004). - PubMed
-
- Shelburne C. P., Abraham S. N., The mast cell in innate and adaptive immunity. Adv. Exp. Med. Biol. 716, 162–185 (2011). - PubMed
-
- Gendrin C., Vornhagen J., Ngo L., Whidbey C., Boldenow E., Santana-Ufret V., Clauson M., Burnside K., Galloway D. P., Waldorf K. M. A., Piliponsky A. M., Rajagopal L., Mast cell degranulation by a hemolytic lipid toxin decreases GBS colonization and infection. Sci. Adv. 1, e1400225 (2015). - PMC - PubMed
-
- St. John A. L., Rathore A. P. S., Yap H., Ng M.-L., Metcalfe D. D., Vasudevan S. G., Abraham S. N., Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc. Natl. Acad. Sci. U.S.A. 108, 9190–9195 (2011). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

