Benefits of Unconventional Methods in the Total Synthesis of Natural Products
- PMID: 30613812
- PMCID: PMC6312638
- DOI: 10.1021/acsomega.8b02994
Benefits of Unconventional Methods in the Total Synthesis of Natural Products
Abstract
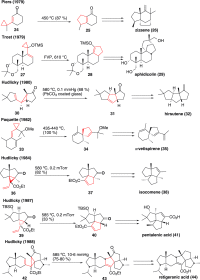
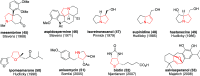
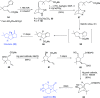
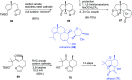
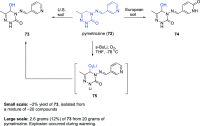
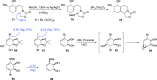
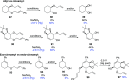
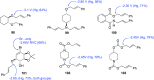
This article provides a survey of four "unconventional" methods employed in the synthesis of natural products in the Hudlicky group. The utility of flash vacuum pyrolysis is highlighted by examples of many natural products attained via vinylcyclopropane-cyclopentene rearrangement and its heterocyclic variants. Preparative organic electrochemistry was used in oxidations and reductions with levels of selectivity unattainable by conventional methods. Yeast reduction of ketoesters was featured in the total synthesis of pyrrolizidine alkaloids. Finally, the use of toluene dioxygenase-mediated dihydroxylations in enantioselective synthesis of natural products concludes this presentation. Recently, synthesized targets in the period 2010-2019 are listed in the accompanying table. The results of research from the Hudlicky group are placed in appropriate context with the work of others, and a detailed guide to the current literature is provided.
Conflict of interest statement
The author declares no competing financial interest.
Figures
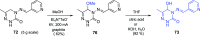


Similar articles
-
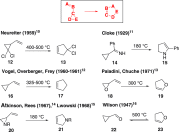
From discovery to application: 50 years of the vinylcyclopropane-cyclopentene rearrangement and its impact on the synthesis of natural products.Angew Chem Int Ed Engl. 2010 Jul 5;49(29):4864-76. doi: 10.1002/anie.200906001. Angew Chem Int Ed Engl. 2010. PMID: 20586104 No abstract available.
-
Non-heme dioxygenase catalyzes atypical oxidations of 6,7-bicyclic systems to form the 6,6-quinolone core of viridicatin-type fungal alkaloids.Angew Chem Int Ed Engl. 2014 Nov 17;53(47):12880-4. doi: 10.1002/anie.201407920. Epub 2014 Sep 22. Angew Chem Int Ed Engl. 2014. PMID: 25251934
-
Total synthesis of pancratistatin relying on the [3,3]-sigmatropic rearrangement.J Org Chem. 2004 Jan 9;69(1):112-21. doi: 10.1021/jo035371n. J Org Chem. 2004. PMID: 14703386
-
Recent applications of the divinylcyclopropane-cycloheptadiene rearrangement in organic synthesis.Beilstein J Org Chem. 2014 Jan 16;10:163-93. doi: 10.3762/bjoc.10.14. eCollection 2014. Beilstein J Org Chem. 2014. PMID: 24605138 Free PMC article. Review.
-
The application of cathodic reductions and anodic oxidations in the synthesis of complex molecules.Chem Soc Rev. 2006 Jul;35(7):605-21. doi: 10.1039/b512308a. Epub 2006 Mar 6. Chem Soc Rev. 2006. PMID: 16791332 Review.
Cited by
-
Concepts and tools for mechanism and selectivity analysis in synthetic organic electrochemistry.Proc Natl Acad Sci U S A. 2019 Jun 4;116(23):11147-11152. doi: 10.1073/pnas.1904439116. Epub 2019 May 17. Proc Natl Acad Sci U S A. 2019. PMID: 31101717 Free PMC article.
-
Bio-click chemistry: a bridge between biocatalysis and click chemistry.RSC Adv. 2022 Jan 12;12(4):1932-1949. doi: 10.1039/d1ra08053a. eCollection 2022 Jan 12. RSC Adv. 2022. PMID: 35425264 Free PMC article. Review.
-
Quantitative 1H-NMR analysis reveals steric and electronic effects on the substrate specificity of benzoate dioxygenase in Ralstonia eutropha B9.J Ind Microbiol Biotechnol. 2022 May 25;49(3):kuac006. doi: 10.1093/jimb/kuac006. J Ind Microbiol Biotechnol. 2022. PMID: 35259264 Free PMC article.
-
Chemical Equivalent of Arene Monooxygenases: Dearomative Synthesis of Arene Oxides and Oxepines.J Am Chem Soc. 2020 Jun 3;142(22):10125-10131. doi: 10.1021/jacs.0c02724. Epub 2020 May 8. J Am Chem Soc. 2020. PMID: 32383862 Free PMC article.
-
Monooxygenase- and Dioxygenase-Catalyzed Oxidative Dearomatization of Thiophenes by Sulfoxidation, cis-Dihydroxylation and Epoxidation.Int J Mol Sci. 2022 Jan 14;23(2):909. doi: 10.3390/ijms23020909. Int J Mol Sci. 2022. PMID: 35055091 Free PMC article. Review.
References
-
-
For leading references to some of these topics see:
- Puri S.; Kaur B.; Parmar A.; Kumar H. Applications of ultrasound in organic synthesis—a green approach. Curr. Org. Chem. 2013, 17, 1790–1828. 10.2174/13852728113179990018. - DOI
- Baig R. B. N.; Varma S. R. Alternative energy input: Mechanochemical, microwave, and ultrasound-assisted organic synthesis. Chem. Soc. Rev. 2012, 41, 1559–1584. 10.1039/C1CS15204A. - DOI - PubMed
- Cravotto G.; Cintas P. Power ultrasound in organic synthesis: Moving cavitational chemistry from academia to innovative and large-scale applications. Chem. Soc. Rev. 2006, 35, 180–196. 10.1039/B503848K. - DOI - PubMed
- Abdulla R. F. Ultrasound in organic synthesis. Aldrichimica Acta 1988, 21, 31–42.
- Jas G.; Kirschning A. Continuous flow techniques in organic synthesis. Chem.- Eur. J. 2003, 9, 5708–5723. 10.1002/chem.200305212. - DOI - PubMed
- Ley S. V. Engineering chemistry for the future of organic synthesis. Tetrahedron 2018, 74, 3087–3100. 10.1016/j.tet.2018.05.046. - DOI
- Ley S. V. The engineering of chemical synthesis: humans and machines working in harmony. Angew. Chem., Int. Ed. 2018, 57, 5182–5183. 10.1002/anie.201802383. - DOI - PubMed
- Movsisyan M.; Delbeke E. I. P.; Berton J. K. E. T.; Battilocchio C.; Ley S. V.; Stevens C. V. Taming hazardous chemistry by continuous flow technology. Chem. Soc. Rev. 2016, 45, 4892–4928. 10.1039/C5CS00902B. - DOI - PubMed
- Ley S. V.; Fitzpatrick D. E.; Myers R. M.; Battilocchio C.; Ingham R. J. Machine-assisted organic synthesis. Angew. Chem., Int. Ed. 2015, 54, 10122–10136. 10.1002/anie.201501618. - DOI - PMC - PubMed
- Pastre J. C.; Browne D. L.; Ley S. V. Flow chemistry syntheses of natural products. Chem. Soc. Rev. 2013, 42, 8849–8869. 10.1039/c3cs60246j. - DOI - PubMed
- Sideri I. K.; Voutyritsa E.; Kokotos C. G. Photoorganocatalysis, small organic molecules and light in the service of organic synthesis: the awakening of a sleeping giant. Org. Biomol. Chem. 2018, 16, 4596–4614. 10.1039/C8OB00725J. - DOI - PubMed
- Eivgi O.; Lemcoff N. G. Turning the light on: Recent developments in photoinduced olefin metathesis. Synthesis 2018, 50, 49–63. 10.1055/s-0036-1589113. - DOI
- Menigaux D.; Belmont P.; Brachet E. Light on unsaturated hydrocarbons—“Gotta heterofunctionalize them all”. Eur. J. Org. Chem. 2017, 15, 2008–2055. 10.1002/ejoc.201601626. - DOI
-
[The entire issue 15 is a special issue on Catalysis]
- Dallinger D.; Kappe C. O. Microwave-assisted synthesis in water as solvent. Chem. Rev. 2007, 107, 2563–2591. 10.1021/cr0509410. - DOI - PubMed
- Stauch T.; Dreuw A. Advances in quantum mechanochemistry: electronic structure methods and force analysis. Chem. Rev. 2016, 116, 14137–14180. 10.1021/acs.chemrev.6b00458. - DOI - PubMed
- Tan D.; Friščić T. Mechanochemistry for organic chemists: an update. Eur. J. Org. Chem. 2018, 18–33. 10.1002/ejoc.201700961. - DOI
- Chanda T.; Zhao J. C.-G. Recent progress in organocatalytic asymmetric domino transformations. Adv. Synth. Catal. 2018, 360, 2–79. 10.1002/adsc.201701059. - DOI
-
-
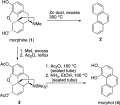
- von Gerichten E.; Schrotter H. Zur Kenntniss des Morphins. Justus Liebigs Ann. Chem. 1881, 210, 396–401. 10.1002/jlac.18812100211. - DOI
-
- Fischer O.; von Gerichten E. Zur Kenntniss des Morphins. Ber. Dtsch. Chem. Ges. 1886, 19, 792–794. 10.1002/cber.188601901180. - DOI
-
-
For reviews of pyrolysis methods see for example:
- Wenrup C. Flash vacuum pyrolysis of azides, triazoles, and tetrazoles. Chem. Rev. 2017, 117, 4562–4623. 10.1021/acs.chemrev.6b00738. - DOI - PubMed
- Wentrup C. Flash vacuum pyrolysis: techniques and reactions. Angew. Chem., Int. Ed. 2017, 56, 14808–14835. 10.1002/anie.201705118. - DOI - PubMed
- Wentrup C. Flash (vacuum) pyrolysis apparatus and methods. Aust. J. Chem. 2014, 67, 1150–1165. 10.1071/CH14096. - DOI
- McNab H. Synthetic applications of flash vacuum pyrolysis. Contemp. Org. Synth. 1996, 3, 373–396. 10.1039/co9960300373. - DOI
- Karpf M. Organic synthesis at high temperatures. Gas-phase flow thermolysis [New synthetic methods (57)]. Angew. Chem., Int. Ed. 1986, 25, 414–430. 10.1002/anie.198604141. - DOI
-
-
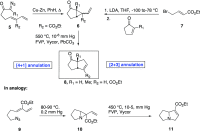
- Hudlicky T.; Rulin F.; Lovelace T. C.; Reed J. W.. Synthesis of Natural Products Containing Five-Membered Rings: An Evolution of General Methodology. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier Science, 1989; Vol. 3, (Part B), , pp 3–72;
- The Way of Synthesis; Hudlicky T., Reed J. W., Eds.; Wiley-VCH, 2007; pp 361–429.
Publication types
LinkOut - more resources
Full Text Sources

