The Neuropeptides of Ocular Immune Privilege, α-MSH and NPY, Suppress Phagosome Maturation in Macrophages
- PMID: 30613828
- PMCID: PMC6319950
- DOI: 10.4049/immunohorizons.1800049
The Neuropeptides of Ocular Immune Privilege, α-MSH and NPY, Suppress Phagosome Maturation in Macrophages
Abstract
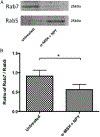
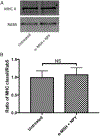
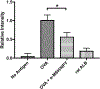
The ocular microenvironment has evolutionarily adapted several mechanisms of immunosuppression to minimize the induction of inflammation. Neuropeptides produced by the retinal pigment epithelial cells regulate macrophage activity. Two neuropeptides, α-melanocyte-stimulating hormone (α -MSH) and neuropeptide Y (NPY), are constitutively expressed by the retinal pigment epithelial cells. Together these two neuropeptides induce anti-inflammatory cytokine production in endotoxin-stimulated macrophages and suppress phagocytosis of unopsonized bioparticles. These neuropeptides do not suppress the phagocytosis of opsonized bioparticles; however, they do suppress phagolysosome activation or formation. In this report, we studied the possibility that α-MSH with NPY suppress phagosome maturation within macrophages using opsonized OVA-coated magnetic beads to isolate and analyze the phagosomes. The magnetic bead-containing intercellular vesicles were isolated and assayed for Rab5, Rab7, LAMP1, Iad, and OVA. The macrophages cotreated with α-MSH and NPY were suppressed in Rab7 recruitment to the phagosome with suppression in LAMP1 expression but not in Iad expression. The results demonstrated that the α-MSH/NPY cotreatment suppressed phagosome maturation. In addition, the a-MSH/NPY-cotreated macrophages were suppressed in their ability to Ag stimulate CD4+ T cell proliferation. These results imply a potential mechanism of ocular immune privilege to divert Ag processing to prevent autoreactive effector T cells from binding their target cognate Ag within the ocular microenvironment.
Conflict of interest statement
DISCLOSURES A.W.T. is a consultant for Palatin Technologies, Cranbury, NJ. The other authors have no financial conflicts of interest.
Figures


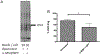

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

