Panitumumab-based maintenance after oxaliplatin discontinuation in metastatic colorectal cancer: A retrospective analysis of two randomised trials
- PMID: 30614531
- PMCID: PMC6590196
- DOI: 10.1002/ijc.32110
Panitumumab-based maintenance after oxaliplatin discontinuation in metastatic colorectal cancer: A retrospective analysis of two randomised trials
Abstract
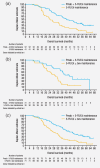
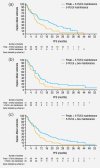
Panitumumab is approved for RAS wild-type metastatic colorectal cancer and was evaluated in Phase III (PRIME, NCT00364013) and Phase II (PEAK, NCT00819780) first-line randomised studies. This retrospective analysis of these trials investigated efficacy and toxicity of panitumumab-based maintenance after oxaliplatin discontinuation in RAS wild-type patients. First-line regimens were FOLFOX4 ± panitumumab in PRIME and mFOLFOX6 plus panitumumab or mFOLFOX6 plus bevacizumab in PEAK. Outcomes included median progression-free survival (PFS) and overall survival (OS), from randomisation and oxaliplatin discontinuation, and toxicity. Overall, median duration of panitumumab plus 5-fluorouracil/leucovorin (5-FU/LV) maintenance was 21 (interquartile range: 11-41) weeks; that of 5-FU/LV ± bevacizumab maintenance was 16 (6-31) weeks. Median OS from randomisation was 40.2 (95% confidence interval: 30.3-50.4) and 39.1 (34.2-63.0) months for panitumumab plus 5-FU/LV maintenance and 24.1 (17.7-33.0) and 28.9 (21.0-32.0) months for 5-FU/LV ± bevacizumab maintenance in PRIME and PEAK, respectively. Median PFS from randomisation was 16.6 (11.3-23.6) and 15.4 (11.6-18.4) months for panitumumab plus 5-FU/LV maintenance and 12.6 (9.4-16.2) and 13.1 (9.5-16.6) months for 5-FU/LV ± bevacizumab maintenance in PRIME and PEAK, respectively. From oxaliplatin discontinuation, median OS was 33.9 (24.7-42.8) and 33.5 (24.5-54.9) months for panitumumab plus 5-FU/LV maintenance and 16.4 (12.4-24.1) and 23.3 (15.7-26.3) months for 5-FU/LV ± bevacizumab maintenance in PRIME and PEAK, respectively; PFS was 11.7 (7.8-19.2) and 9.7 (5.8-14.8) months and 7.1 (5.6-10.2) and 7.0 (3.9-10.6) months, respectively. The most frequently reported adverse events were rash, fatigue and diarrhoea. Maintenance of panitumumab plus 5-FU/LV after oxaliplatin discontinuation was well tolerated and may be an acceptable treatment paradigm for patients demonstrating a good response to first-line treatment. Prospective studies are warranted.
Keywords: epidermal growth factor receptor; maintenance; metastatic colorectal cancer; panitumumab; survival; toxicity.
© 2019 The Authors. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Figures
References
-
- European Medicines Agency . Summary of product characteristics. Vectibix 20 mg/mL concentrate for solution for infusion. 2016 [cited 2018 Nov 8]; Available from: https://www.medicines.org.uk/emc/medicine/20528
-
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–422. - PubMed
-
- Douillard JY, Oliner KS, Siena S, et al. Panitumumab‐FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023–34. - PubMed
-
- Rivera F, Karthaus M, Hecht JR, et al. Final analysis of the randomised PEAK trial: overall survival and tumour responses during first‐line treatment with mFOLFOX6 plus either panitumumab or bevacizumab in patients with metastatic colorectal carcinoma. Int J Colorectal Dis 2017;32:1179–90. - PMC - PubMed
-
- Schwartzberg LS, Rivera F, Karthaus M, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild‐type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol 2014;32:2240–7. - PubMed

