Modelling continuous abstinence rates over time from clinical trials of pharmacological interventions for smoking cessation
- PMID: 30614586
- PMCID: PMC6492005
- DOI: 10.1111/add.14549
Modelling continuous abstinence rates over time from clinical trials of pharmacological interventions for smoking cessation
Abstract
Background and aim: It is useful, for theoretical and practical reasons, to be able to specify functions for continuous abstinence over time in smoking cessation attempts. This study aimed to find the best-fitting models of mean proportion abstinent with different smoking cessation pharmacotherapies up to 52 weeks from the quit date.
Methods: We searched the Cochrane Database of Systematic Reviews to identify randomized controlled trials (RCTs) of pharmacological treatments to aid smoking cessation. For comparability, we selected trials that provided 12 weeks of treatment. Continuous abstinence rates for each treatment at each follow-up point in trials were extracted along with methodological details of the trial. Data points for each pharmacotherapy at each follow-up point were aggregated where the total across contributing studies included at least 1000 participants per data point. Continuous abstinence curves were modelled using a range of different functions from the quit date to 52-week follow-up. Models were compared for fit using R2 and Bayesian information criterion (BIC).
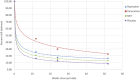
Results: Studies meeting our selection criteria covered three pharmacotherapies [varenicline, nicotine replacement therapy (NRT) and bupropion] and placebo. Power functions provided the best fit (R2 > 0.99, BIC < 17.0) to continuous abstinence curves from the target quit date in all cases except for varenicline, where a logarithmic function described the curve best (R2 = 0.99, BIC = 21.2). At 52 weeks, abstinence rates were 22.5% (23.0% modelled) for varenicline, 16.7% (16.0% modelled) for bupropion, 13.0% (12.4% modelled) for NRT and 8.3% (8.9% modelled) for placebo. For varenicline, bupropion, NRT and placebo, respectively, 55.9, 65.0, 62.3 and 56.5% of participants who were abstinent at the end of treatment were still abstinent at 52 weeks.
Conclusions: Mean continuous abstinence rates up to 52 weeks from initiation of smoking cessation attempts in clinical trials can be modelled using simple power functions for placebo, nicotine replacement therapy and bupropion and a logarithmic function for varenicline. This allows accurate prediction of abstinence rates from any time point to any other time point up to 52 weeks.
Keywords: Bupropion; continuous abstinence; nicotine replacement therapy; pharmacological interventions; relapse; smoking cessation; smoking cessation aids; varenicline.
© 2019 The Authors. Addiction published by John Wiley & Sons Ltd on behalf of Society for the Study of Addiction.
Figures

References
-
- Hughes J. R., Keely J., Naud S. Shape of the relapse curve and long‐term abstinence among untreated smokers. Addiction 2004; 99: 29–38. - PubMed
-
- Anthenelli R. M., Benowitz N. L., West R., Aubin L. S., McRae T., Lawrence D. et al Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double‐blind, randomised, placebo‐controlled clinical trial. Lancet 2016; 387: 2507–2520. - PubMed
-
- Tønnesen P., Paoletti P., Gustavsson G., Russell M. A., Saracci R., Gulsvik A. et al Higher dosage nicotine patches increase one‐year smoking cessation rates: results from the European CEASE trial. Collaborative European anti‐smoking evaluation. European Respiratory Society. Eur Respir J 1999; 13: 238–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

