Long-term efficacy and safety of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: 104-week VERTIS MET trial
- PMID: 30614616
- PMCID: PMC6593724
- DOI: 10.1111/dom.13631
Long-term efficacy and safety of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: 104-week VERTIS MET trial
Abstract
Aim: To evaluate the long-term efficacy and safety of ertugliflozin in adults with type 2 diabetes mellitus inadequately controlled on metformin.
Materials and methods: A 104-week Phase III, randomized double-blind study with a 26-week placebo-controlled period (Phase A) and a 78-week period (Phase B) where blinded glimepiride was added to non-rescued placebo participants with fasting fingerstick glucose ≥6.1 mmol/L. Results through week 104 are reported.
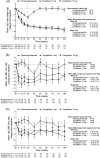
Results: Mean (standard error) change in HbA1c from baseline was -0.7% (0.07) and -1.0% (0.07) at week 52; -0.6% (0.08) and -0.9% (0.08) at week 104 for ertugliflozin 5 and 15 mg. At week 52, 34.8% and 36.6% participants had HbA1c <7.0%, and 24.6% and 33.7% at week 104, for ertugliflozin 5 and 15 mg. Ertugliflozin reduced fasting plasma glucose (FPG), body weight and systolic blood pressure (SBP) from baseline through week 104. The incidence of female genital mycotic infections (GMIs) was higher with ertugliflozin, and symptomatic hypoglycaemia was lower for ertugliflozin versus placebo/glimepiride. Minimal bone mineral density (BMD) changes were observed, similar to placebo/glimepiride, except at total hip where reduction in BMD was greater with ertugliflozin 15 mg versus placebo/glimepiride: difference in least squares means (95% CI) -0.50% (-0.95, -0.04) at week 52 and -0.84% (-1.44, -0.24) at week 104.
Conclusions: Ertugliflozin maintained improvements from baseline in HbA1c, FPG, body weight and SBP through week 104. Ertugliflozin was well tolerated, with non-clinically relevant changes in BMD. Compared with placebo/glimepiride, ertugliflozin increased female GMIs, but reduced the incidence of symptomatic hypoglycaemia. ClinicalTrials.gov Identifier: NCT02033889.
Keywords: bone mineral density; durability; ertugliflozin; type 2 diabetes mellitus.
© 2019 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
Author contributions
All authors critically reviewed the draft manuscript and approved the final version of the manuscript for publication. A. D., S. G. T. and B. L. were involved in the conception/design of the study. S. G., A. G., H. S. and A. D. were involved in the acquisition of data for the study. All authors were involved in data analysis and interpretation of the data.
Data accessibility
Upon request, and subject to certain criteria, conditions and exceptions (see
Figures



References
-
- Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733‐794. - PubMed
-
- Scheen AJ. Pharmacokinetics, pharmacodynamics and clinical use of SGLT2 inhibitors in patients with type 2 diabetes mellitus and chronic kidney disease. Clin Pharmacokinet. 2015;54:691‐708. - PubMed
-
- Kalgutkar AS, Tugnait M, Zhu T, et al. Preclinical species and human disposition of PF‐04971729, a selective inhibitor of the sodium‐dependent glucose cotransporter 2 and clinical candidate for the treatment of type 2 diabetes mellitus. Drug Metab Dispos. 2011;39:1609‐1619. - PubMed
-
- Miao Z, Nucci G, Amin N, et al. Pharmacokinetics, metabolism, and excretion of the antidiabetic agent ertugliflozin (PF‐04971729) in healthy male subjects. Drug Metab Dispos. 2013;41:445‐456. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

